1. Gero D, Gié O, Hübner M, Demartines N, Hahnloser D. 2017; Postoperative ileus: in search of an international consensus on definition, diagnosis, and treatment. Langenbecks Arch Surg. 402:149–158. DOI:
10.1007/s00423-016-1485-1. PMID:
27488952.

3. Li YY, Cao MH, Goetz B, Chen CQ, Feng YJ, Chen CJ, Kasparek MS, Sibaev A, Storr M, Kreis ME. 2013; The dual effect of cannabinoid receptor-1 deficiency on the murine postoperative ileus. PLoS One. 8:e67427. DOI:
10.1371/journal.pone.0067427. PMID:
23844009. PMCID:
PMC3701010.

4. Stakenborg N, Gomez-Pinilla PJ, Boeckxstaens GE. 2017; Postoperative Ileus: pathophysiology, current therapeutic approaches. Handb Exp Pharmacol. 239:39–57. DOI:
10.1007/164_2016_108. PMID:
27999957.

5. Rychter J, Clavé P. 2013; Intestinal inflammation in postoperative ileus: pathogenesis and therapeutic targets. Gut. 62:1534–1535. DOI:
10.1136/gutjnl-2012-304176. PMID:
23396510.

8. Farro G, Gomez-Pinilla PJ, Di Giovangiulio M, Stakenborg N, Auteri M, Thijs T, Depoortere I, Matteoli G, Boeckxstaens GE. 2016; Smooth muscle and neural dysfunction contribute to different phases of murine postoperative ileus. Neurogastroenterol Motil. 28:934–947. DOI:
10.1111/nmo.12796. PMID:
26891411.

9. Stakenborg N, Labeeuw E, Gomez-Pinilla PJ, De Schepper S, Aerts R, Goverse G, Farro G, Appeltans I, Meroni E, Stakenborg M, Viola MF, Gonzalez-Dominguez E, Bosmans G, Alpizar YA, Wolthuis A, D'Hoore A, Van Beek K, Verheijden S, Verhaegen M, Derua R, et al. 2019; Preoperative administration of the 5-HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut. 68:1406–1416. DOI:
10.1136/gutjnl-2018-317263. PMID:
30472681. PMCID:
PMC6691854.

10. Endo M, Hori M, Ozaki H, Oikawa T, Hanawa T. 2014; Daikenchuto, a traditional Japanese herbal medicine, ameliorates postoperative ileus by anti-inflammatory action through nicotinic acetylcholine receptors. J Gastroenterol. 49:1026–1039. DOI:
10.1007/s00535-013-0854-6. PMID:
23846546. PMCID:
PMC4048467.

11. Kalff JC, Buchholz BM, Eskandari MK, Hierholzer C, Schraut WH, Simmons RL, Bauer AJ. 1999; Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery. 126:498–509. DOI:
10.1016/S0039-6060(99)70091-7. PMID:
10486602.

12. Wehner S, Schwarz NT, Hundsdoerfer R, Hierholzer C, Tweardy DJ, Billiar TR, Bauer AJ, Kalff JC. 2005; Induction of IL-6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery. 137:436–446. DOI:
10.1016/j.surg.2004.11.003. PMID:
15800492.

14. Kreiss C, Birder LA, Kiss S, VanBibber MM, Bauer AJ. 2003; COX-2 dependent inflammation increases spinal Fos expression during rodent postoperative ileus. Gut. 52:527–534. DOI:
10.1136/gut.52.4.527. PMID:
12631664. PMCID:
PMC1773580.

15. Kalff JC, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. 2000; Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 118:316–327. DOI:
10.1016/S0016-5085(00)70214-9. PMID:
10648460.

16. Turan A, Celik I. 2016; Antioxidant and hepatoprotective properties of dried fig against oxidative stress and hepatotoxicity in rats. Int J Biol Macromol. 91:554–559. DOI:
10.1016/j.ijbiomac.2016.06.009. PMID:
27268385.

17. Fujita H, Fujishima H, Chida S, Takahashi K, Qi Z, Kanetsuna Y, Breyer MD, Harris RC, Yamada Y, Takahashi T. 2009; Reduction of renal superoxide dismutase in progressive diabetic nephropathy. J Am Soc Nephrol. 20:1303–1313. DOI:
10.1681/ASN.2008080844. PMID:
19470681. PMCID:
PMC2689908.

18. Liu W, Xu Z, Li H, Guo M, Yang T, Feng S, Xu B, Deng Y. 2017; Protective effects of curcumin against mercury-induced hepatic injuries in rats, involvement of oxidative stress antagonism, and Nrf2-ARE pathway activation. Hum Exp Toxicol. 36:949–966. DOI:
10.1177/0960327116677355. PMID:
27837179.

19. Esterbauer H, Cheeseman KH. 1990; Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 186:407–421. DOI:
10.1016/0076-6879(90)86134-H. PMID:
2233308.
20. Valenzuela A. 1991; The biological significance of malondialdehyde determination in the assessment of tissue oxidative stress. Life Sci. 48:301–309. DOI:
10.1016/0024-3205(91)90550-U. PMID:
1990230.

23. Odaka T, Suzuki T, Seza A, Yamaguchi T, Saisho H. 2006; [Serotonin 5- HT4 receptor agonist (mosapride citrate)]. Nihon Rinsho. 64:1491–1494. Japanese. PMID:
16898619.
24. Tsuchida Y, Hatao F, Fujisawa M, Murata T, Kaminishi M, Seto Y, Hori M, Ozaki H. 2011; Neuronal stimulation with 5-hydroxytryptamine 4 receptor induces anti-inflammatory actions via α7nACh receptors on muscularis macrophages associated with postoperative ileus. Gut. 60:638–647. DOI:
10.1136/gut.2010.227546. PMID:
21115544. PMCID:
PMC3071096.

25. Toyomasu Y, Mochiki E, Morita H, Ogawa A, Yanai M, Ohno T, Fujii T, Tsutsumi S, Asao T, Kuwano H. 2011; Mosapride citrate improves postoperative ileus of patients with colectomy. J Gastrointest Surg. 15:1361–1367. DOI:
10.1007/s11605-011-1567-x. PMID:
21607794.

27. Balza E, Piccioli P, Carta S, Lavieri R, Gattorno M, Semino C, Castellani P, Rubartelli A. 2016; Proton pump inhibitors protect mice from acute systemic inflammation and induce long-term cross-tolerance. Cell Death Dis. 7:e2304. DOI:
10.1038/cddis.2016.218. PMID:
27441656. PMCID:
PMC4973356.

28. Okamoto A, Kohama K, Aoyama-Ishikawa M, Yamashita H, Fujisaki N, Yamada T, Yumoto T, Nosaka N, Naito H, Tsukahara K, Iida A, Sato K, Kotani J, Nakao A. 2016; Intraperitoneally administered, hydrogen-rich physiologic solution protects against postoperative ileus and is associated with reduced nitric oxide production. Surgery. 160:623–631. DOI:
10.1016/j.surg.2016.05.026. PMID:
27425040.

29. Murakami H, Li S, Foreman R, Yin J, Hirai T, Chen JDZ. 2019; Intraoperative vagus nerve stimulation accelerates postoperative recovery in rats. J Gastrointest Surg. 23:320–330. DOI:
10.1007/s11605-018-3969-5. PMID:
30264388.

30. Nam Y, Lee JM, Wang Y, Ha HS, Sohn UD. 2016; The effect of Flos Lonicerae Japonicae extract on gastro-intestinal motility function. J Ethnopharmacol. 179:280–290. DOI:
10.1016/j.jep.2015.12.056. PMID:
26743226.

31. Barquist E, Zinner M, Rivier J, Taché Y. 1992; Abdominal surgery-induced delayed gastric emptying in rats: role of CRF and sensory neurons. Am J Physiol. 262(4 Pt 1):G616–G620. DOI:
10.1152/ajpgi.1992.262.4.G616. PMID:
1566844.

32. Asgeirsson T, El-Badawi KI, Mahmood A, Barletta J, Luchtefeld M, Senagore AJ. 2010; Postoperative ileus: it costs more than you expect. J Am Coll Surg. 210:228–231. DOI:
10.1016/j.jamcollsurg.2009.09.028. PMID:
20113944.

33. Tevis SE, Carchman EH, Foley EF, Harms BA, Heise CP, Kennedy GD. 2015; Postoperative ileus--more than just prolonged length of stay? J Gastrointest Surg. 19:1684–1690. DOI:
10.1007/s11605-015-2877-1. PMID:
26105552.

34. Kalff JC, Carlos TM, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. 1999; Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 117:378–387. DOI:
10.1053/gast.1999.0029900378. PMID:
10419919.

36. Ellozy SH, Harris MT, Bauer JJ, Gorfine SR, Kreel I. 2002; Early postoperative small-bowel obstruction: a prospective evaluation in 242 consecutive abdominal operations. Dis Colon Rectum. 45:1214–1217. DOI:
10.1007/s10350-004-6395-6. PMID:
12352239.
37. Murakami H, Li S, Foreman R, Yin J, Hirai T, Chen JDZ. 2019; Ameliorating effects of electroacupuncture on dysmotility, inflammation, and pain mediated via the autonomic mechanism in a rat model of postoperative ileus. J Neurogastroenterol Motil. 25:286–299. DOI:
10.5056/jnm18094. PMID:
30827069. PMCID:
PMC6474706.

38. Stoffels B, Hupa KJ, Snoek SA, van Bree S, Stein K, Schwandt T, Vilz TO, Lysson M, Veer CV, Kummer MP, Hornung V, Kalff JC, de Jonge WJ, Wehner S. 2014; Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology. 146:176–187.e1. DOI:
10.1053/j.gastro.2013.09.030. PMID:
24067878.

39. van Bree SH, Nemethova A, Cailotto C, Gomez-Pinilla PJ, Matteoli G, Boeckxstaens GE. 2012; New therapeutic strategies for postoperative ileus. Nat Rev Gastroenterol Hepatol. 9:675–683. DOI:
10.1038/nrgastro.2012.134. PMID:
22801725.

40. Xiong YJ, Chu HW, Lin Y, Han F, Li YC, Wang AG, Wang FJ, Chen DP, Wang JY. 2016; Hesperidin alleviates rat postoperative ileus through anti-inflammation and stimulation of Ca
2+-dependent myosin phosphorylation. Acta Pharmacol Sin. 37:1091–1100. DOI:
10.1038/aps.2016.56. PMID:
27345626. PMCID:
PMC4973386.
41. Łuszczak J, Ziaja-Sołtys M, Rzymowska J. 2011; Anti-oxidant activity of superoxide dismutase and glutathione peroxidase enzymes in skeletal muscles from slaughter cattle infected with Taenia saginata. Exp Parasitol. 128:163–165. DOI:
10.1016/j.exppara.2011.01.007. PMID:
21272584.

42. Wang Y, Branicky R, Noë A, Hekimi S. 2018; Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 217:1915–1928. DOI:
10.1083/jcb.201708007. PMID:
29669742. PMCID:
PMC5987716.

43. Özkan N, Ersoy ÖF, Özsoy Z, Çakır E. 2018; Melatonin exhibits supportive effects on oxidants and anastomotic healing during intestinal ischemia/reperfusion injury. Ulus Travma Acil Cerrahi Derg. 24:1–8. DOI:
10.5505/tjtes.2017.23539. PMID:
29350377.
44. Qin S, McLaughlin AP, De Vries GW. 2006; Protection of RPE cells from oxidative injury by 15-deoxy-delta12,14-prostaglandin J2 by augmenting GSH and activating MAPK. Invest Ophthalmol Vis Sci. 47:5098–5105. DOI:
10.1167/iovs.06-0318. PMID:
17065531.
45. Lawrence L, Menon S, Vincent S, Sivaram VP, Padikkala J. 2016; Radical scavenging and gastroprotective activity of methanolic extract of Gmelina arborea stem bark. J Ayurveda Integr Med. 7:78–82. DOI:
10.1016/j.jaim.2016.06.003. PMID:
27449207. PMCID:
PMC4969311.

46. Akinrinmade FJ, Akinrinde AS, Soyemi OO, Oyagbemi AA. 2016; Antioxidant potential of the methanol extract of Parquetina nigrescens mediates protection against intestinal ischemia-reperfusion injury in rats. J Diet Suppl. 13:420–432. DOI:
10.3109/19390211.2015.1103828. PMID:
26634775.
47. da Silva LM, Boeing T, Somensi LB, Cury BJ, Steimbach VM, Silveria AC, Niero R, Cechinel Filho V, Santin JR, de Andrade SF. 2015; Evidence of gastric ulcer healing activity of Maytenus robusta Reissek: in vitro and in vivo studies. J Ethnopharmacol. 175:75–85. DOI:
10.1016/j.jep.2015.09.006. PMID:
26364940.
48. Zhuang CL, Chen FF, Lu JX, Zheng BS, Liu S, Zhou CJ, Huang DD, Shen X, Yu Z. 2015; Impact of different surgical traumas on postoperative ileus in rats and the mechanisms involved. Int J Clin Exp Med. 8:16778–16786. PMID:
26629220. PMCID:
PMC4659108.
49. Den Boer PJ, Poot M, Verkerk A, Jansen R, Mackenbach P, Grootegoed JA. 1990; Glutathione-dependent defence mechanisms in isolated round spermatids from the rat. Int J Androl. 13:26–38. DOI:
10.1111/j.1365-2605.1990.tb00957.x. PMID:
2312188.

50. Chen FF, Zhou CJ, Zhuang CL, Huang DD, Lu JX, Shen X, Chen XL, Yu Z. 2015; Mitochondrial energy metabolism disorder and apoptosis: a potential mechanism of postoperative ileus. Int J Clin Exp Med. 8:14885–14895. PMID:
26628970. PMCID:
PMC4658859.
51. De Backer O, Elinck E, Blanckaert B, Leybaert L, Motterlini R, Lefebvre RA. 2009; Water-soluble CO-releasing molecules reduce the development of postoperative ileus via modulation of MAPK/HO-1 signalling and reduction of oxidative stress. Gut. 58:347–356. DOI:
10.1136/gut.2008.155481. PMID:
19022916.

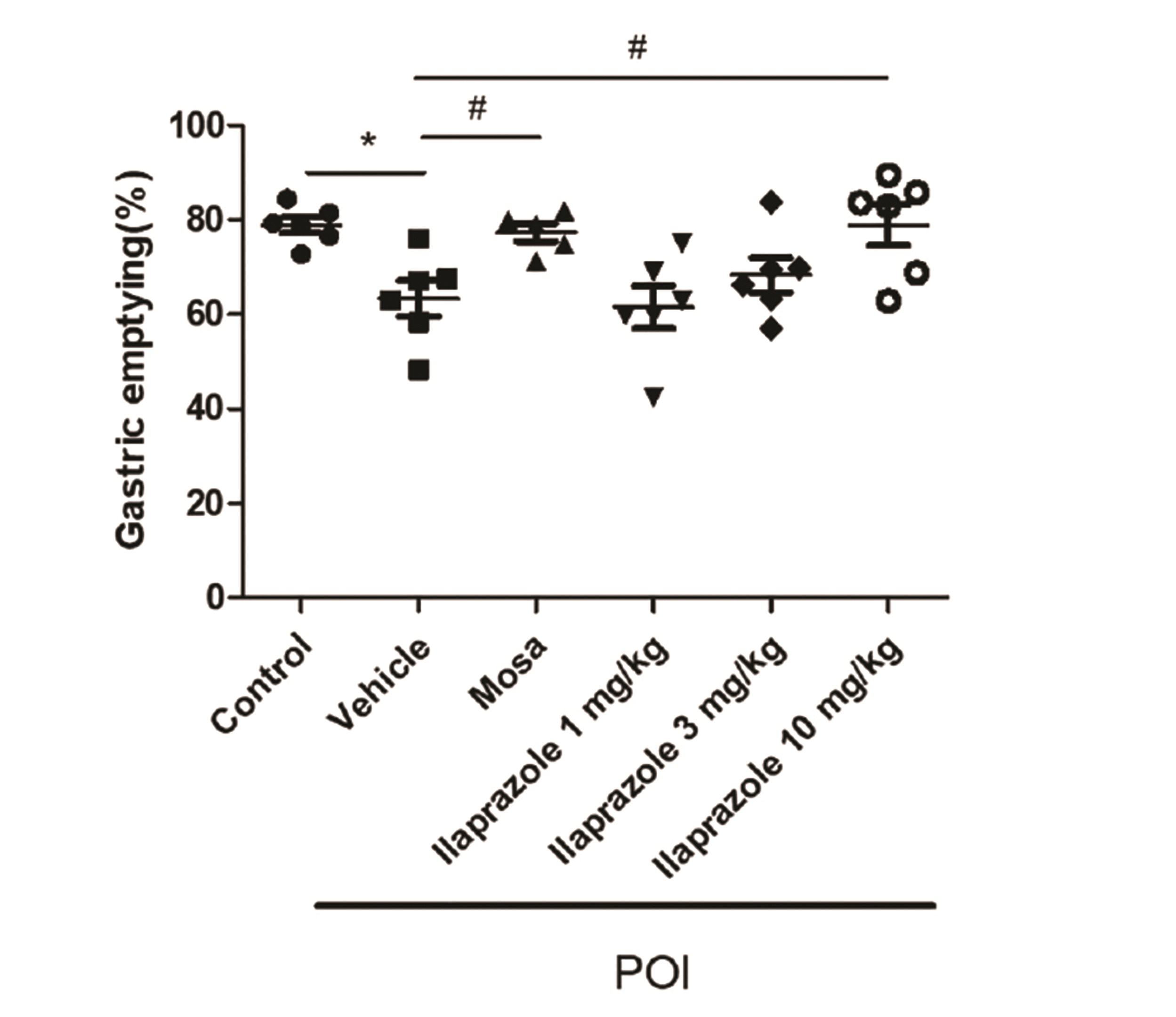
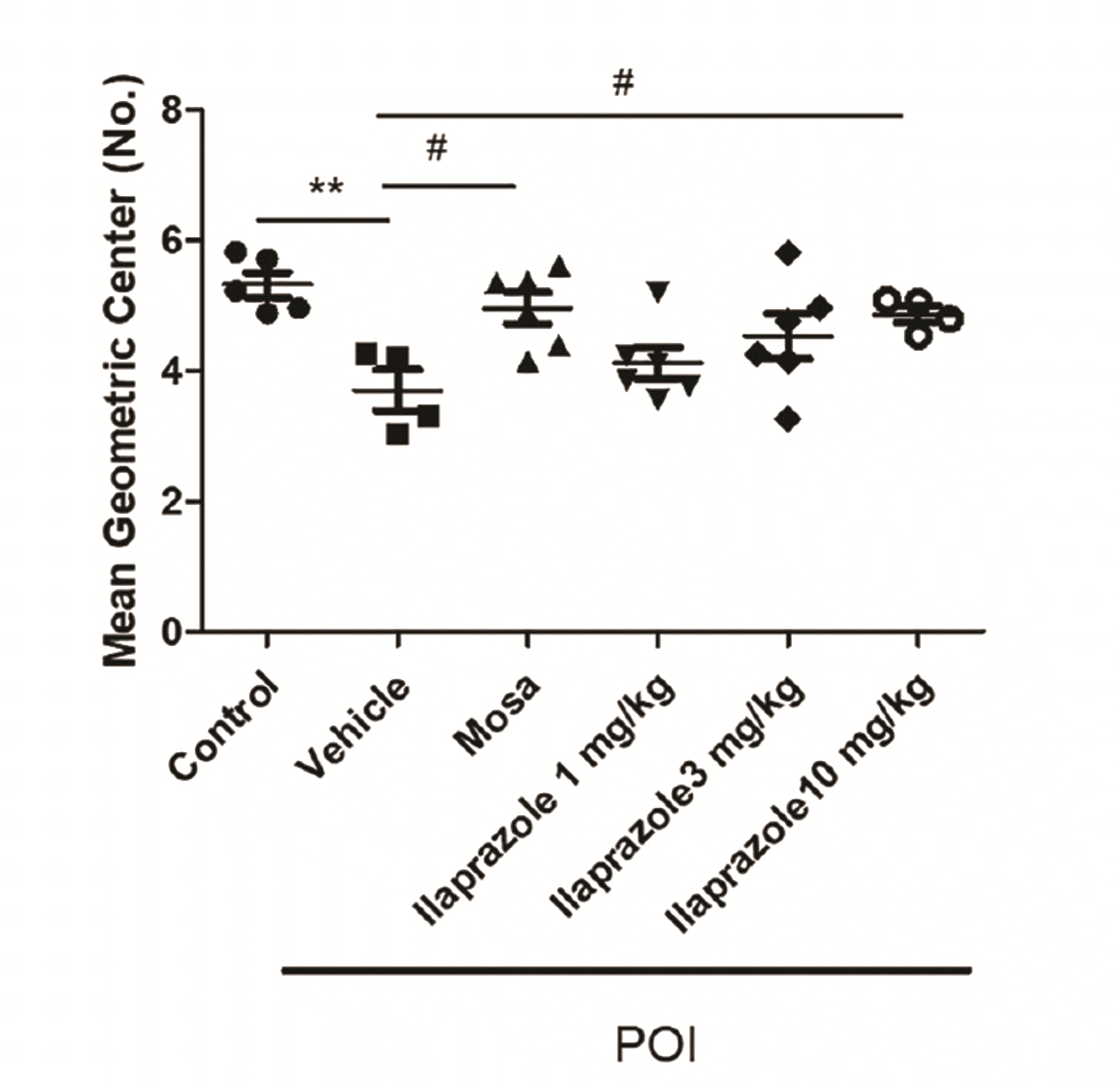
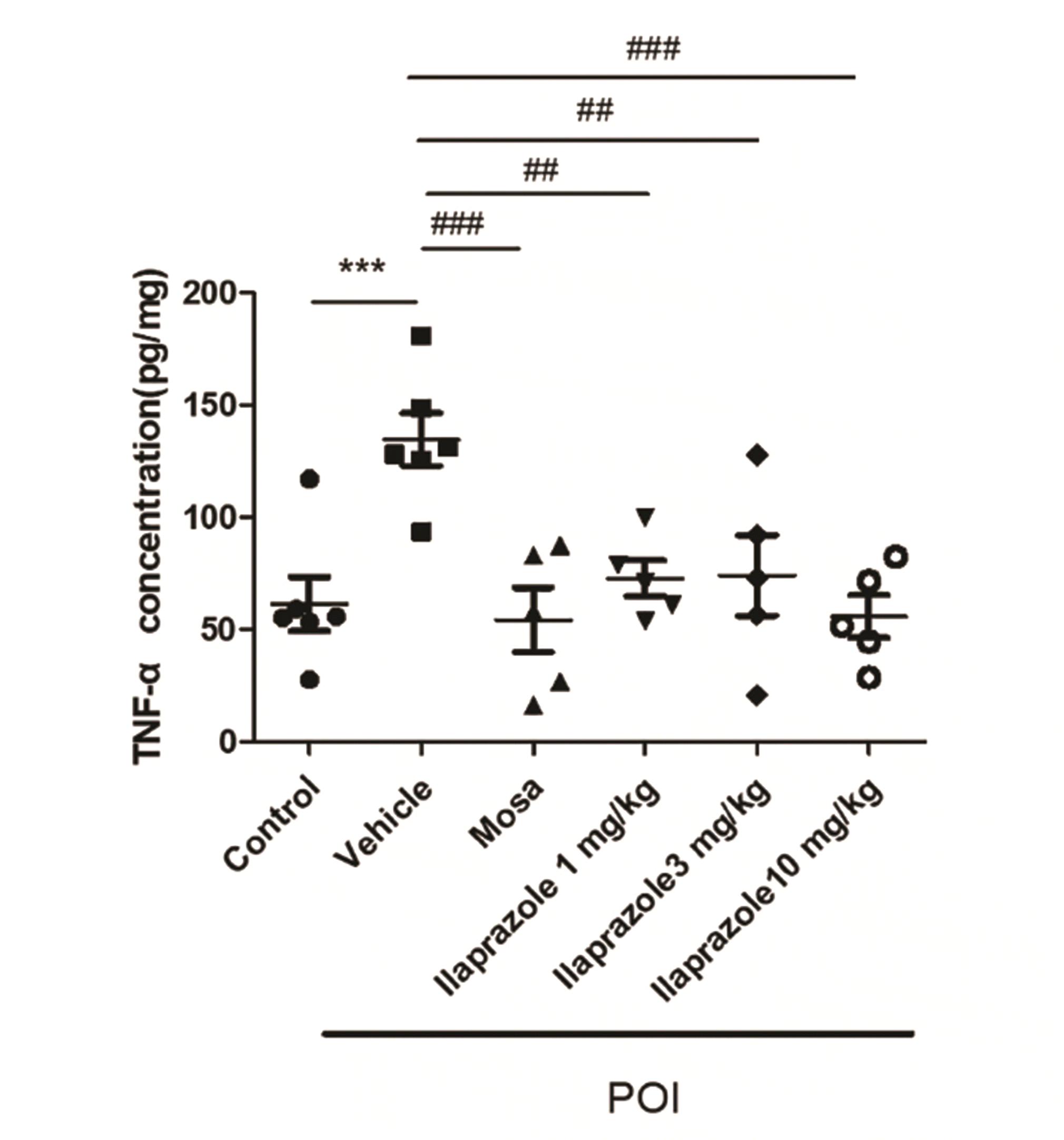
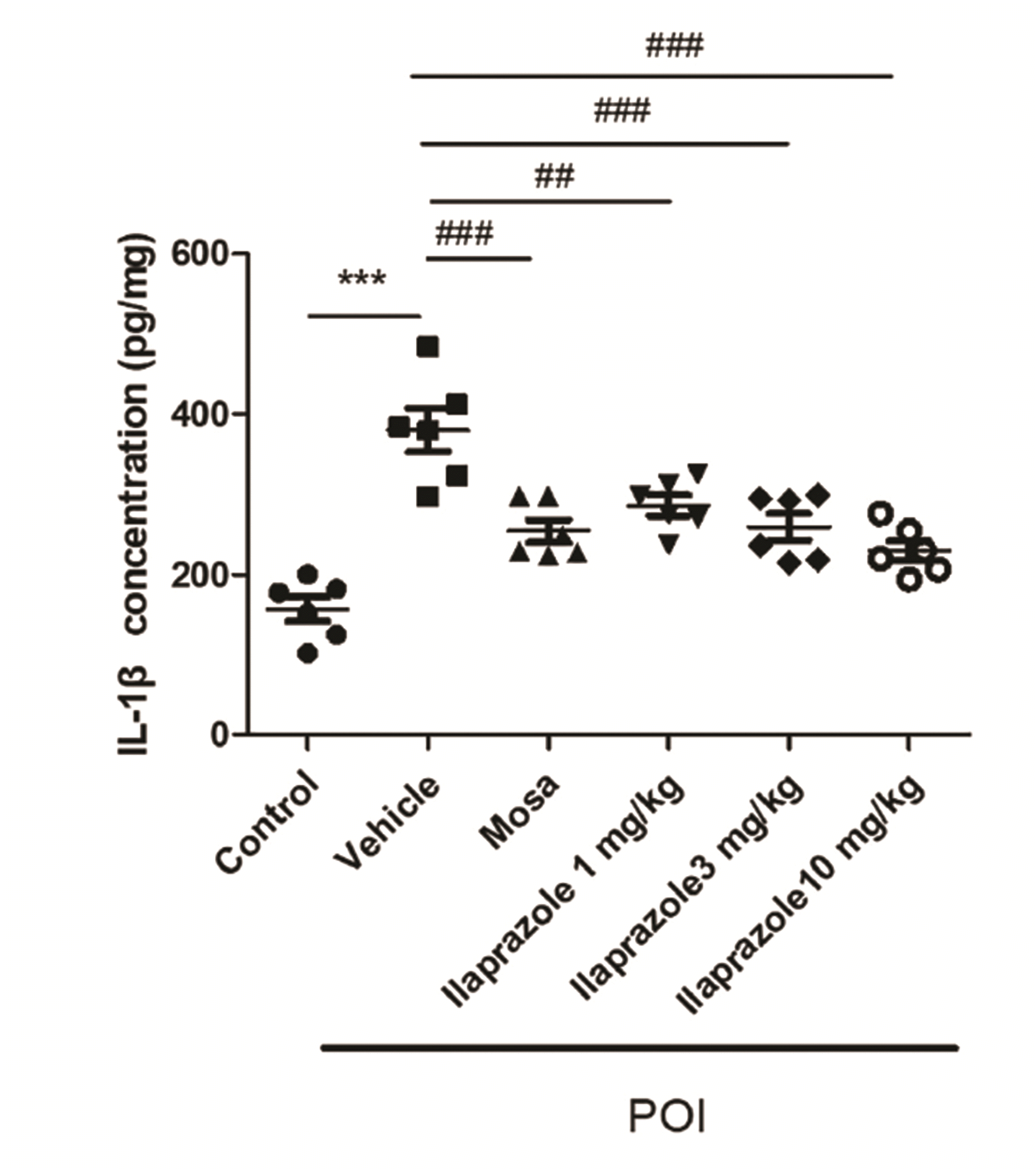





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download