Abstract
Preoperative imatinib treatment for rectal gastrointestinal stromal tumors (GISTs) has been reported to reduce the tumor size and help preserve the anal sphincter function. On the other hand, preoperative imatinib may prevent an accurate assessment of the recurrent risk. The endoscopic resection of rectal GIST is rarely reported because of challenges that include securing the visual field and avoiding perforation. This paper reports a case in which a 5.5×4.0 cm sized rectal GIST was treated effectively by an endoscopic submucosal dissection (ESD) without preoperative imatinib. To date, the patient had no tumor recurrence or complications and is receiving adjuvant imatinib treatment. This case shows that ESD may be a good treatment option to preserve the anus in rectal GIST treatment.
Gastrointestinal stromal tumors (GISTs) are mainly found as submucosal tumors resulting from mutations in the KIT or platelet-derived growth factor receptor protooncogenes in the interstitial cells of Cajal.1 GISTs are the most common mesenchymal tumors of the gastrointestinal tract. A surgical resection with a negative margin is the standard treatment for patients with primary GISTs. The treatment of rectal GISTs should consider the sphincter-sparing approach.2,3 On the other hand, there is no consensus regarding the amount and frequency of imatinib administration before surgery that allows the preservation of the anal sphincter. Preoperative imatinib may prevent an accurate assessment of the recurrent risk. Endoscopic resection is a novel treatment for rectal GIST that allows complete resection of the anal sphincter, but it is rarely reported. This paper reports the successful endoscopic resection of a huge rectal GIST without the administration of preoperative imatinib.
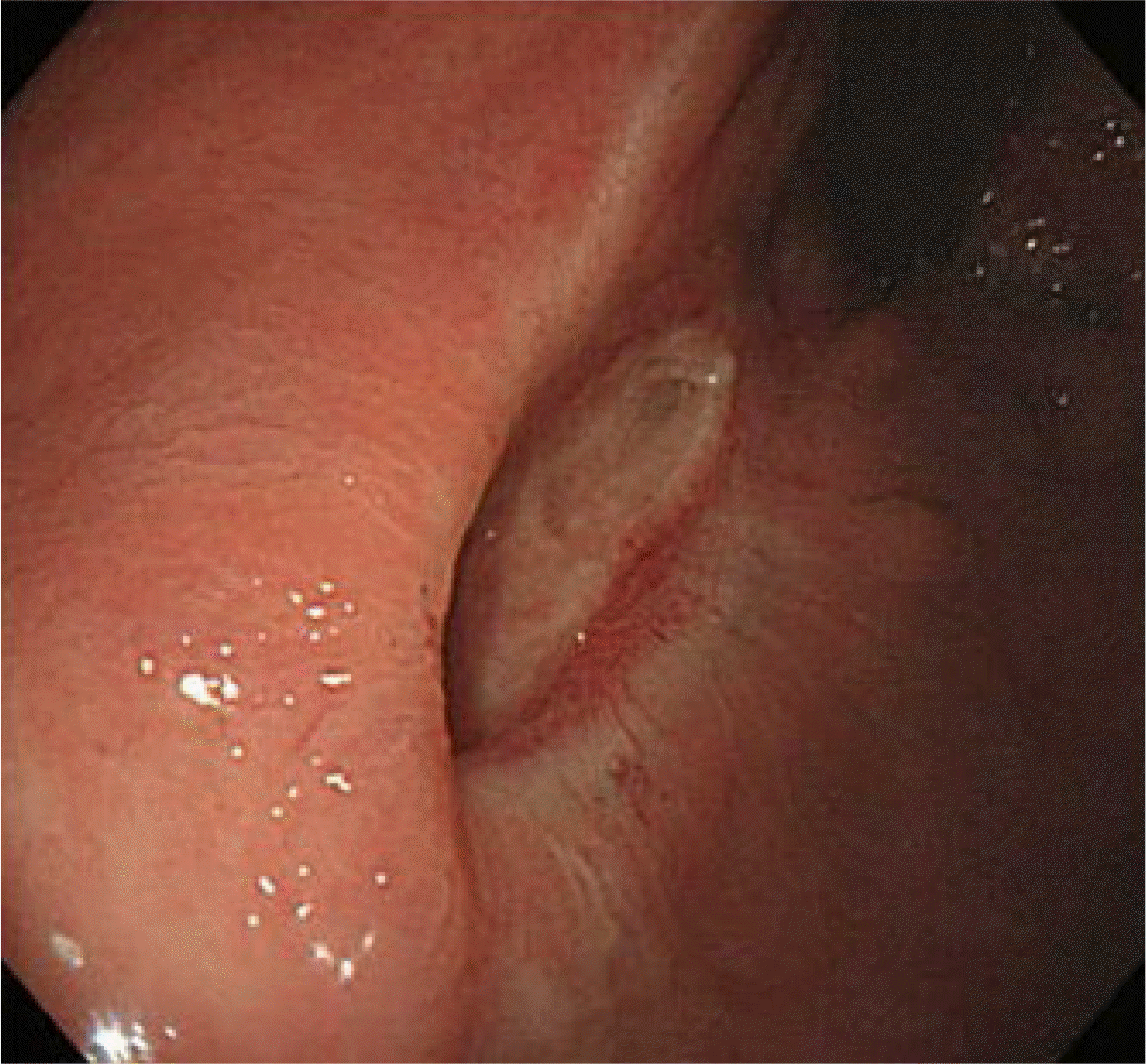
A 71-year-old man presented with recurrent constipation. He had no previous illnesses or family history. A digital rectal examination revealed a hard mass in the distal rectum. Colonoscopy revealed a huge submucosal tumor, approximately 5 cm in diameter, in the distal rectum involving the anal canal. Endoscopic ultrasonography revealed a 5.5×4.0 cm heterogeneous hypoechoic mass originating from the muscularis propria. Chest and abdominal computed tomography showed a 5.5×4.0 cm sized round mass of soft tissue density in the distal rectum without local or distant metastases (Fig. 1). The patient was presented with two treatment plans: imatinib treatment after surgical removal or preoperative imatinib treatment followed by surgery. The patient preferred removal first but was concerned about the possibility of anal sphincter injury. An endoscopic resection was performed to preserve the anal sphincter function using a single-channel waterjet upper gastrointestinal endoscope (GIF H290, Olympus, Tokyo, Japan) with a short-type ST hood (DH-28GR, Fujifilm, Tokyo, Japan) under deep sedation (midazolam 3 mg+propofol 20 mg, 20 mg added after consciousness evaluation per 20-30 sec). After injection with a mixture of glycerol, epinephrine, indigo carmine, and normal saline, Dual knife (KD-650Q, Olympus) and IT-knife nano (KD-612L/U, Olympus) were used to incise the mucosa and dissect the submucosal and proper muscle layer in the proximal direction, starting from the anal side (Fig. 2). The following procedures were performed: Endocut Q mode (effect 3, duration 2, interval 3) for incision, swift coagulation current (effect 3, 40 W) for dissection, and soft coagulation current (effect 3, 60 W) for hemostasis. The base of the tumor was located in the proper muscle layer and was excised completely by dissection to the inner circular muscle layer. The duration of the procedure was 110 min. The histopathology examination revealed a 5.5×4.0 cm sized, well-defined, encapsulated mass consisting of spindle and focally epithelioid cells arranged diffuse pattern from the mucosa to the circular layer of the proper muscle. The tumor cells were positive for KIT (CD117), CD34, and DOG1. More than 20 mitoses per 50 high-power fields were noted, corresponding to a high risk of malignant GIST recurrence (Fig. 3). The patient was prescribed oral imatinib (400 mg/day). Three months after surgery, a colonoscopy revealed a small ulcer and healed scar without recurrence (Fig. 4). To date, the patient is receiving imatinib treatment without tumor recurrence or complications.
Rectal GIST requires a multidisciplinary approach to determine the best treatment for complete tumor resection and preservation of the anal sphincter. Preoperative imatinib should be considered if a patient with rectal GIST undergoes an abdominoperineal resection.2,3 Although preoperative imatinib treatment can reduce the tumor size and increase the complete resection rate, it has some limitations. First, it reduces the tumor size and mitotic rate, making an accurate assessment of the risk of recurrence difficult. Therefore, preoperative imatinib should be considered only if the tumor can be downstaged. Second, there is controversy regarding the actual improvement in survival rates.4,5 Third, testing for mutations before imatinib administration is recommended to identify the genotypes likely to respond to treatment.6 Tumors without a genotype likely to respond to treatment cannot receive preoperative imatinib. Fourth, the maximum duration of the treatment response is not known. The guidelines recommend 6 months or longer, but the exact duration of treatment cannot be predicted in some patients.2,3
The standard treatment for GIST without distant metastases is complete surgical resection with a microscopically negative margin. Generally, minimally invasive, local resection without lymphadenectomy is performed because of the low incidence of lymph node metastasis. Similarly, transanal endoscopic microsurgery (TEM) is commonly used in rectal GISTs to preserve the anal sphincter function.7,8 On the other hand, TEM must be performed under either general or spinal anesthesia and requires high-cost equipment and a long hospital stay. A colorectal endoscopic resection is a novel procedure that enables the en bloc resection of colorectal tumors. An endoscopic resection can be performed under conscious or deep sedation, without anesthesia.
In a meta-analysis of rectal tumor treatment, endoscopic submucosal dissection had similar resection rates, side effects, and recurrence rates to TEM, but shorter treatment and hospitalization periods.9 Similar results were confirmed when comparing only rectal epithelial tumors in our previous retrospective study.10 There is a major limitation in reflecting the overall subepithelial tumor because the registered sample size of the study was small, and most of the registered patients were small neuroendocrine tumors.10 Therefore, future studies on the endoscopic treatment of rectal subepithelial tumors are needed.
While an endoscopic resection of gastric GISTs is relatively common, colorectal GISTs have rarely been reported.11 The reason for this is the relatively thin colon wall, which increases the risk of perforation. On the other hand, as the rectum is in the pelvic cavity, complications from perforation are exceedingly rare. Therefore, a more aggressive endoscopic deep resection, including the muscle layer, is possible. To date, there is no consensus regarding the maximum size of rectal GISTs that can be removed with an endoscopic resection. A huge rectal GIST (>5 cm) was removed by endoscopic resection without neoadjuvant imatinib.
Despite some limitations in securing the visual field and complications, such as bleeding or perforation, advances in endoscopic techniques and equipment are expected to increase the endoscopic treatment of rectal GISTs. Currently, there is no precise recommendation for the endoscopic resection of rectal GISTs. On the other hand, if further efforts to perform endoscopic treatment continue, endoscopic resection may be a suitable treatment option for rectal GISTs. In conclusion, an endoscopic resection should be considered for the treatment of rectal GIST as an anal sphincter preserving approach.
ACKNOWLEDGEMENT
The authors thank the patient, who consented to having his data published in this case report.
REFERENCES
1. Fletcher CD, Berman JJ, Corless C, et al. 2002; Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 33:459–465. DOI: 10.1053/hupa.2002.123545. PMID: 12094370.

2. von Mehren M, Randall RL, Benjamin RS, et al. 2018; Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 16:536–563. DOI: 10.6004/jnccn.2018.0025. PMID: 29752328.

3. Koo DH, Ryu MH, Kim KM, et al. 2016; Asian consensus guidelines for the diagnosis and management of gastrointestinal stromal tumor. Cancer Res Treat. 48:1155–1166. DOI: 10.4143/crt.2016.187. PMID: 27384163. PMCID: PMC5080813.

4. Blesius A, Cassier PA, Bertucci F, et al. 2011; Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial. BMC Cancer. 11:72. DOI: 10.1186/1471-2407-11-72. PMID: 21324142. PMCID: PMC3052196.

5. Eisenberg BL, Harris J, Blanke CD, et al. 2009; Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 99:42–47. DOI: 10.1002/jso.21160. PMID: 18942073. PMCID: PMC2606912.

6. Patrikidou A, Domont J, Chabaud S, et al. 2016; Long-term outcome of molecular subgroups of GIST patients treated with standard-dose imatinib in the BFR14 trial of the French Sarcoma Group. Eur J Cancer. 52:173–180. DOI: 10.1016/j.ejca.2015.10.069. PMID: 26687836.

7. Han X, Xu J, Qiu H, Lin G. 2017; A novel curative treatment strategy for patients with lower grade rectal gastrointestinal stromal tumor: chemoreduction combined with transanal endoscopic microsurgery. J Laparoendosc Adv Surg Tech A. 27:579–585. DOI: 10.1089/lap.2017.0051. PMID: 28358587.

8. Liu Q, Zhong G, Zhou W, Lin G. 2017; Initial application of transanal endoscopic microsurgery for high-risk lower rectal gastrointestinal stromal tumor after imatinib mesylate neoadjuvant chemotherapy: a case report. Medicine (Baltimore). 96:e7538. DOI: 10.1097/MD.0000000000007538. PMID: 28723770. PMCID: PMC5521910.
9. McCarty TR, Bazarbashi AN, Hathorn KE, Thompson CC, Aihara H. 2020; Endoscopic submucosal dissection (ESD) versus transanal endoscopic microsurgery (TEM) for treatment of rectal tumors: a comparative systematic review and meta-analysis. Surg Endosc. 34:1688–1695. DOI: 10.1007/s00464-019-06945-1. PMID: 31292744.

10. Jung Y, Lee J, Cho JY, et al. 2018; Comparison of efficacy and safety between endoscopic submucosal dissection and transanal endoscopic microsurgery for the treatment of rectal tumor. Saudi J Gastroenterol. 24:115–121. DOI: 10.4103/sjg.SJG_440_17. PMID: 29637919. PMCID: PMC5900471.

11. Konuma H, Fu K, Konuma I, et al. 2011; A rectal GI stromal tumor completely resected with endoscopic submucosal dissection (with video). Gastrointest Endosc. 73:1322–1325. DOI: 10.1016/j.gie.2010.09.017. PMID: 21111411.

Fig. 1
Initial colonoscopy and endoscopic ultrasonography. (A) Rectal retroflection during colonoscopy shows an approximately 5 cm-sized submucosal tumor-like lesion between the first Houston plate of rectum and anus. (B) The distal margin of the lesion was extremely close to the anus in the forward endoscopic view. (C) Endoscopic ultrasonography showed that 5.5×4.0 cm sized heterogeneous hypoechoic mass originated from muscularis propria. (D) Computed tomography showed a 5.5×4.0 cm sized round mass of soft tissue density in the distal rectum.
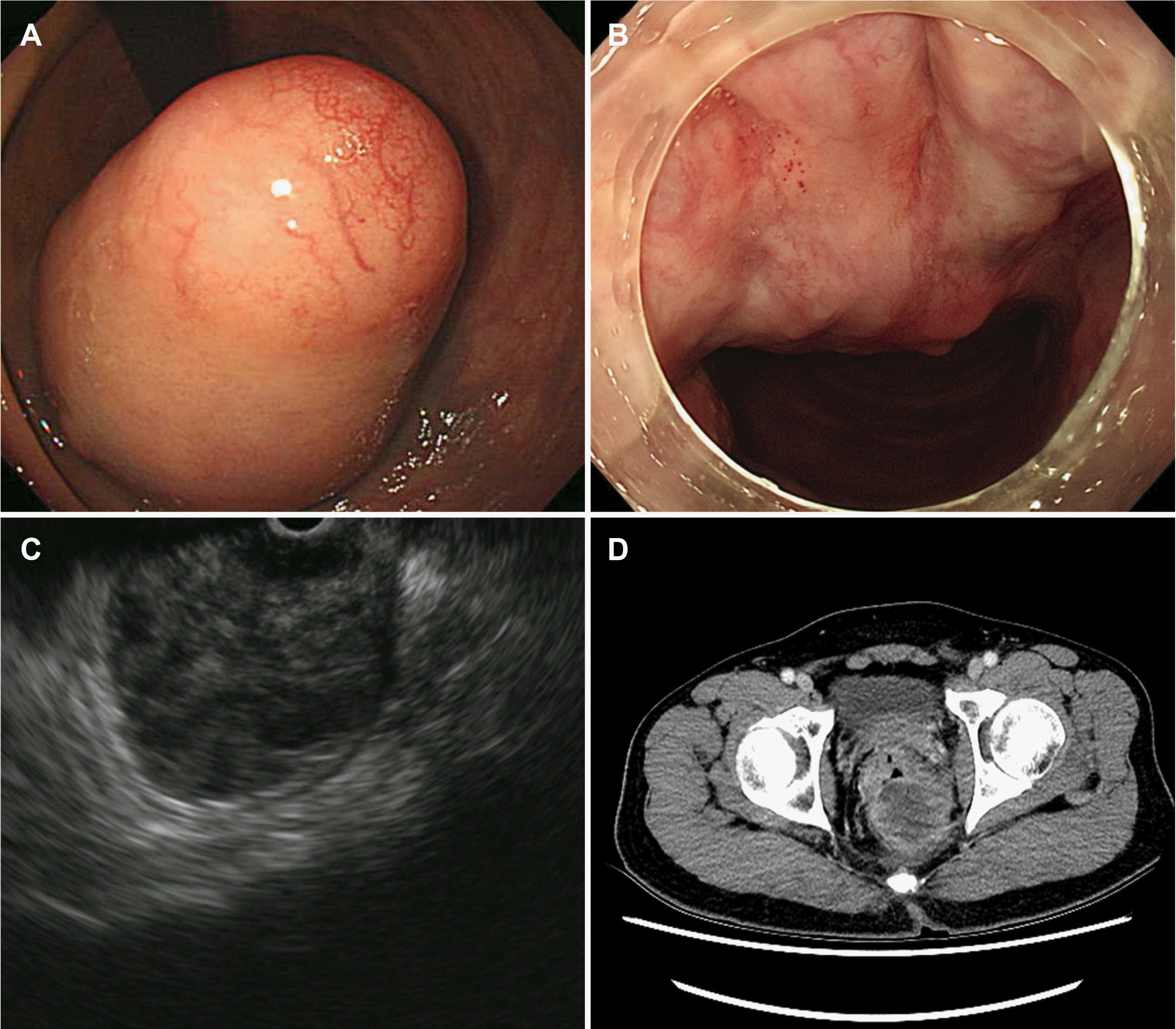
Fig. 2
Endoscopic resection. (A) After submucosal injection, the mucosal incision starts on the anal side of the lesion using a Dual knife. (B) The proper muscle layer dissection was performed using Dual knife and IT knife nano. (C) The rectal mass lesion was removed completely without complications. (D) The 5.5×4.0 cm sized specimen with the mass lesion after its en bloc resection.
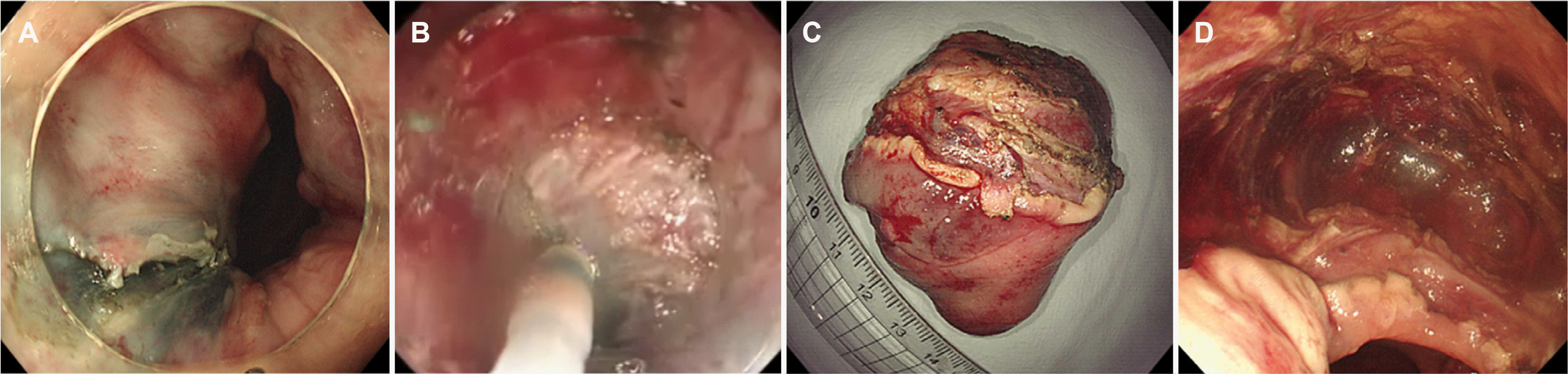
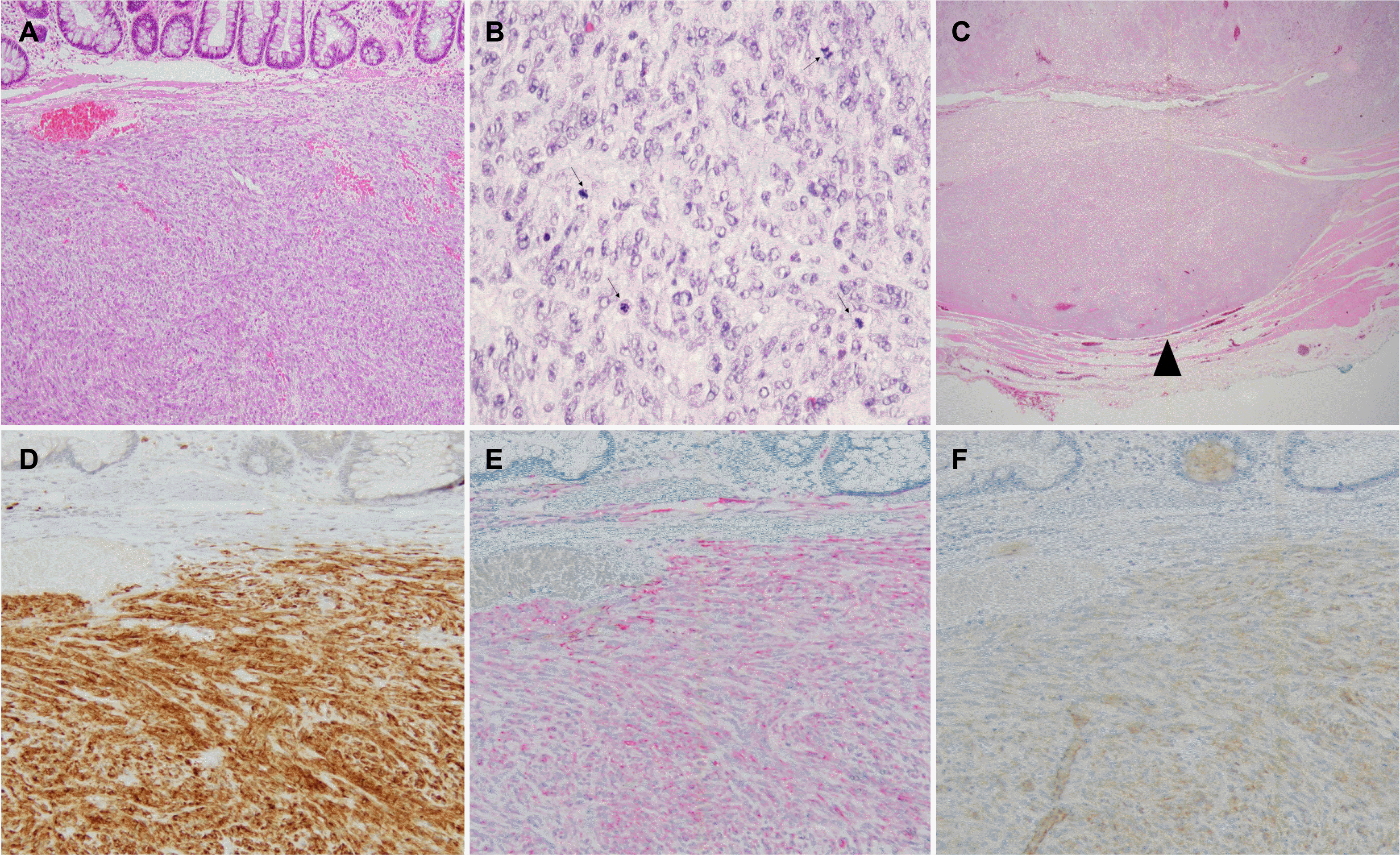
Fig. 3
Pathological findings of the tumor and immunohistochemical staining. The tumor is located from the mucosa to the circular layer of proper muscle. (A) Proliferating round to oval-shaped cells dissecting the muscularis mucosa (Hematoxylin and Eosin [H&E] staining, ×100) and (B) show frequent mitosis (arrow, H&E staining, ×200). (C) The deepest resection margin of the specimen was free of the lesion (arrowhead, H&E staining, ×40). Immunohistochemically, the tumor cells were reactive for (D) c-Kit, (E) CD34 , and (F) DOG1 (respectively, ×200).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download