Abstract
Gastric cancer (GC) is the second most common malignancy in Korea. Most deaths are due to disease recurrence; hence, efforts have been made to identify the recurrence patterns and establish a therapeutic strategy for GC. A 56-year-old man was diagnosed with signet ring cell carcinoma (SRCC) of the stomach 26 years ago, in December 1992. He had local recurrence in November 2006. The patient visited a hospital for back pain in April 2018. Laboratory tests, computed tomography (CT) scans, bone scan, bone and bone marrow biopsies, and bone marrow aspiration were conducted. Bone scan revealed hotspots indicating tracer uptake in the ribs, sternum, pelvic bones, and multiple vertebrae. Chest and abdominal CT scans revealed no lesion except diffuse osteosclerotic lesions of the bony thorax. Finally, bone marrow studies indicated metastatic SRCC. The patient survived for 26 months after treatment with four regimens of palliative chemotherapy. Each regimen was changed due to disease progression. He died due to pneumonia in June 2020. Metastasis to the bone and bone marrow in GC with a disease-free interval of more than 10 years is extremely rare. The patient had an unusually long survival time compared to that reported in previous studies, which have shown a median survival time of less than 3 months. To the best of our knowledge, in Korea, this is the first case of metastasis of gastric SRCC to the bone and bone marrow with a disease-free interval of more than 10 years.
Go to : 
초록
위암은 한국에서 두 번째로 흔한 악성종양으로 알려져 있다. 대부분의 사망은 재발로 인한 것이기 때문에 위암에 대한 치료계획을 수립하기 위해서는 재발양상의 분석이 필요하다. 56세 남성은 26년 전인 1992년 12월에 위반지세포암종 진단을 받았고, 2006년 11월 국소 재발하였다. 환자는 2018년 4월 허리통증으로 병원을 방문하였고 혈액검사, 컴퓨터단층촬영, 뼈스캔, 골 및 골수 생검, 골수천자를 실시하였다. 뼈스캔에서는 갈비뼈, 흉골, 골반뼈 및 여러 척추의 흡수를 보였으며 흉부 및 복부 CT 스캔에서 흉추의 골경화성 병변을 제외하고 다른 병변은 없었다. 최종적으로 골수검사에서 반지세포암종이 진단되었다. 환자는 26개월 동안 생존하면서 4가지 완화 화학요법으로 치료받았고 질병의 진행이 확인되어 각 화학요법이 변경되었다. 환자는 2020년 6월 폐렴으로 사망했다. 위암에서 무병기간이 10년 이상인 골수와 골수로의 전이는 극히 드물고 예후가 좋지 않다. 환자는 이전 연구에서 중앙생존시간이 3개월 미만이었던 것과는 대조적으로 긴 생존 시간을 보였다. 우리가 아는 한, 이번 사례는 국내에서 무병 간격이 10년 이상이고 골수 전이 사진이 있는 골과 골수로의 위 반지세포암종 전이의 첫 번째 사례이다.
Go to : 
Korea has the highest incidence rate of gastric cancer (GC) in females and the second-highest incidence rate in males worldwide [1]. Furthermore, GC is the second most common cancer in Korea [2]. Since recurrence of GC is the major cause of GC-related deaths, efforts have been made to identify the patterns of recurrence and establish appropriate therapeutic strategies [3]. A recurrence after more than 10 years is rare, and the most common site for GC recurrence is the peritoneum [4, 5]. We present the case of a 56-year-old man who was diagnosed with metastasis of gastric signet ring cell carcinoma (SRCC) to only the bone and bone marrow, with a 12-year disease-free interval (DFI) after local recurrence in 2006, which was 14 years after the first diagnosis in 1992.
Go to : 
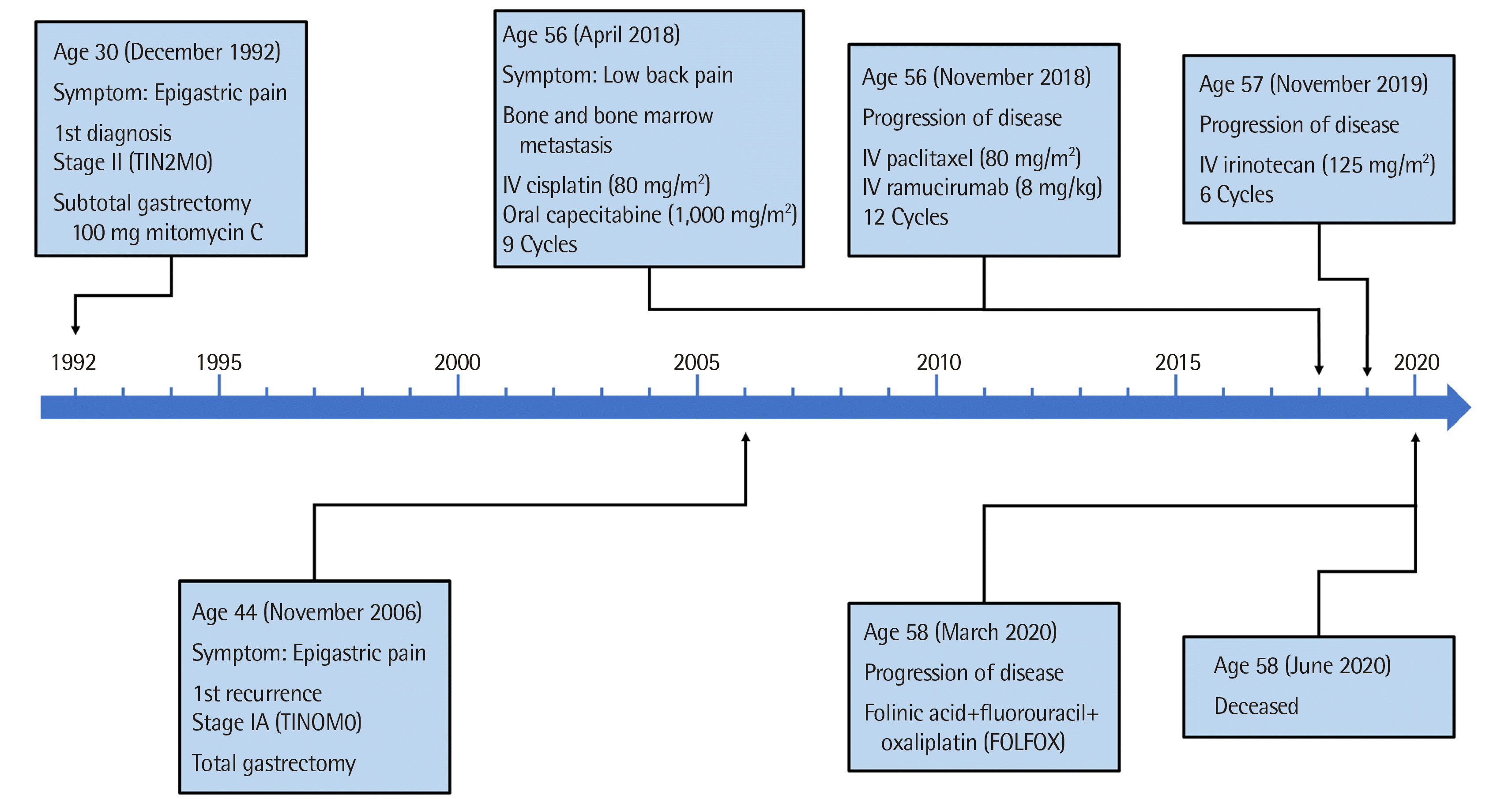
In December 1992, a 30-year-old man visited our hospital with complaints of epigastric pain. Gastrofibroscopy (GFS) and biopsy of an ulcer at the angle of the lesser curvature were performed. The findings revealed the presence of adenocarcinoma with poorly differentiated, signet ring cells. He underwent subtotal gastrectomy with Billroth II gastrojejunostomy. Tumor node metastasis (TNM) classification, according to the 4th edition of the American Joint Committee on Cancer (AJCC) staging system [6], was stage II (T1N2M0). After the surgery, he was treated with a total dose of 100 mg of intravenous (IV) mitomycin C over 10 cycles. His follow-up surveillance, which included GFS and tumor markers such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), showed no evidence of recurrence until 1996. In 2005, he revisited our hospital due to epigastric pain. GFS, computed tomography (CT), and laboratory tests were conducted and showed no evidence of recurrence at that time. In 2006, he underwent a follow-up GFS and biopsy in the stomal area, which revealed SRCC. Total gastrectomy with Roux-en-Y anastomosis was then performed. A second TNM classification of his tumor, according to the 5th edition of the AJCC staging system [7], was stage IA (T1N0M0). No other treatment was provided after surgery, and annual follow-up examinations revealed no evidence of recurrence until 2015.
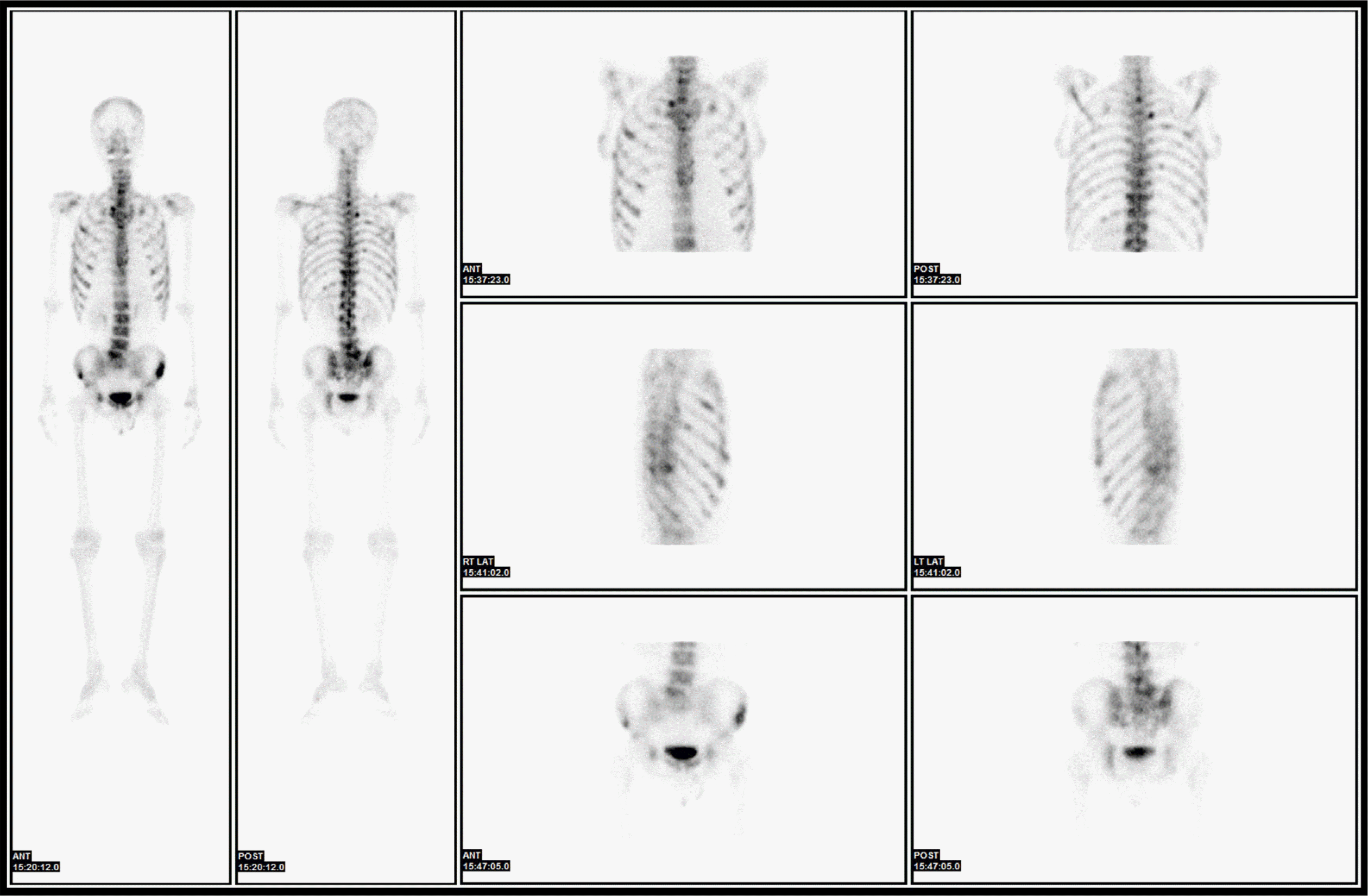
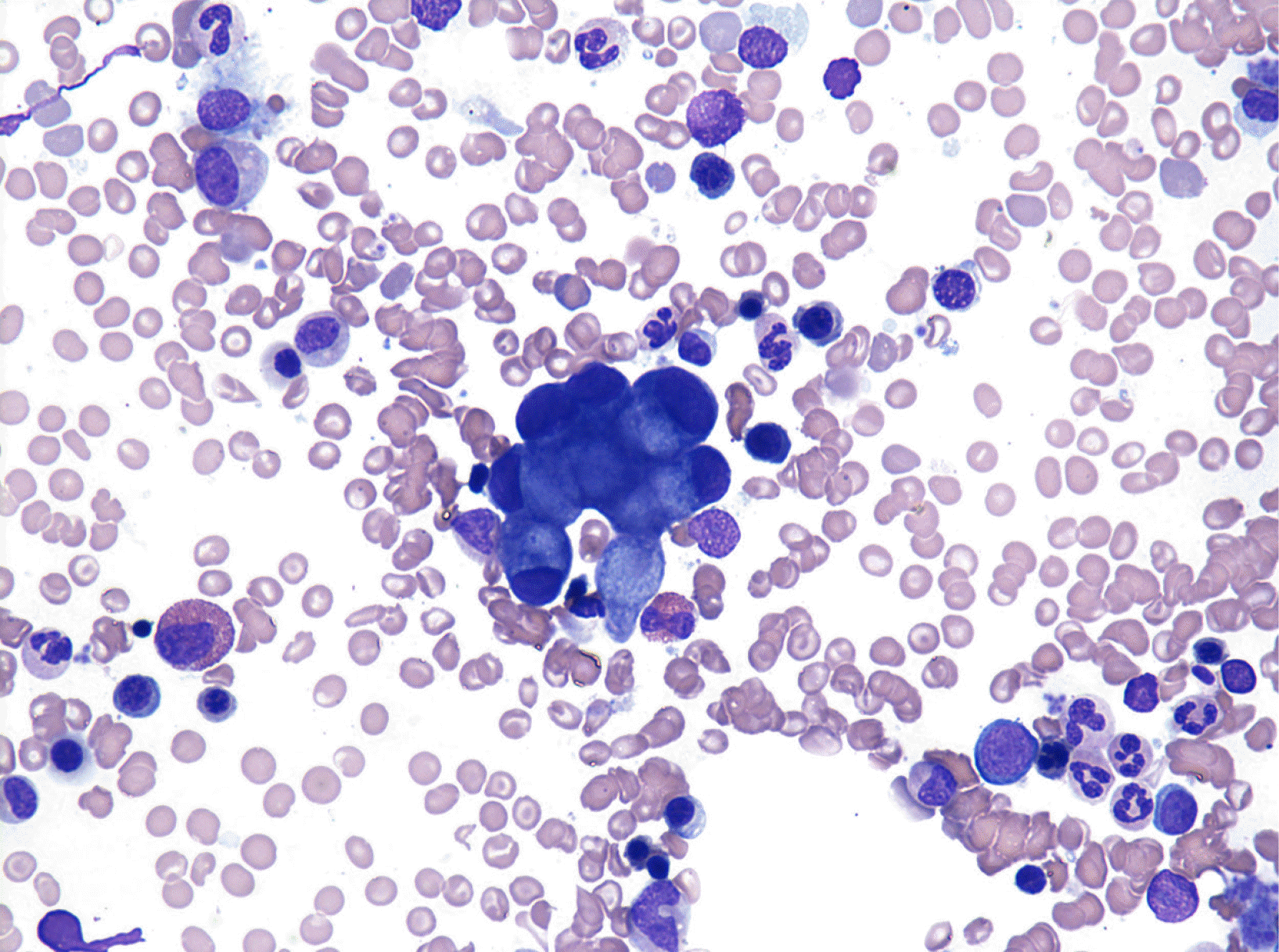
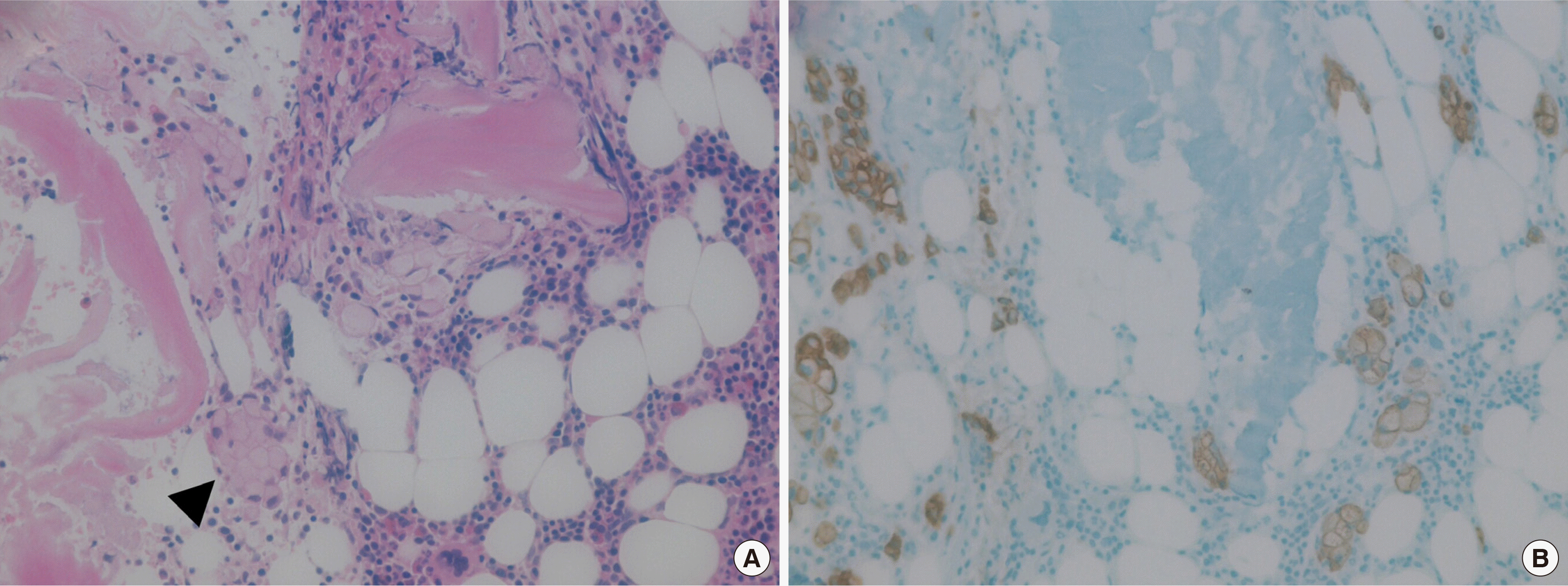
In 2018, the patient, now 56 years old, visited our hospital with a complaint of back pain. He underwent magnetic resonance imaging and CT. The findings suggested multiple myeloma or metastasis to multiple sites. He was hospitalized, and a complete blood count revealed a hemoglobin level of 9.6 g/dL, a white blood cell count of 3×109/L, and a platelet count of 273×109/L. Red blood cell count, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration were 4.05×106/μL, 31%, 76.5 fL, 23.7 pg, and 31 g/dL, respectively. Laboratory findings were suggestive of iron-deficiency anemia and pernicious anemia due to decreased levels of serum iron, at 36 μg/dL (normal range, 70–190 μg/dL), ferritin, at 6.86 ng/mL (normal range, 29–278 ng/mL), and vitamin B12, at 64.68 pg/mL (normal range, 160–970 pg/mL), and increased levels of total iron-binding capacity of 495 μg/dL (normal range, 260–400 μg/dL). The results of the biochemistry tests were within the normal range, except for a decreased serum calcium level of 7.9 mg/dL (normal range, 8.6–10.6 mg/dL) and an increased alkaline phosphatase (ALP) level of 435 IU/L (normal range, 30–120 IU/L). Hypocalcemia and an elevated ALP level are likely to be associated with increased osteoblastic activity. The levels of CEA and CA19-9 tumor markers were normal. GFS, which was performed again, showed non-specific findings, and chest and abdominal CT scans revealed no lesion except diffuse osteosclerotic lesions of the bony thorax. Bone scan showed hotspots indicating tracer uptake in the ribs, sternum, pelvic bones, and multiple vertebrae (Fig. 1). Bone and bone marrow biopsies and bone marrow aspiration were performed. Bone marrow aspirate smears showed normocellular marrow with abnormal cells that were occasionally seen alone or in clusters (Fig. 2). They were large-sized cells with eccentrically placed nuclei and abundant cytoplasm. Bone marrow biopsy stained with hematoxylin and eosin (HE) revealed metastatic signet ring cell carcinoma in the normal marrow space (Fig. 3). Immunohistochemical (IHC) staining of the bone marrow biopsy was positive for pan-cytokeratin antibody and cytokeratin 7 antibody (CK7) but negative for cytokeratin 20 antibody (CK20). Based on the results of the bone marrow biopsy and the patient’s clinical history, signet ring carcinoma was strongly believed to have originated from the GC.
Chemotherapy was initiated. During cycles 1-9, the patient had been prescribed IV cisplatin (80 mg/m2) on day 1 and oral capeci-tabine (1,000 mg/m2) on days 1-14. Unfortunately, a 6-month follow-up positron emission tomography-CT (PET-CT) scan conducted in November 2018 showed increased fluorodeoxyglucose uptake in the sacral area, and disease progression was suspected. The patient’s regimen was then changed to IV paclitaxel (80 mg/m2) on days 1, 8, and 15 with IV ramucirumab (8 mg/kg) administered on days 1 and 15. After 12 cycles of the second regimen, progressive metabolic disease was observed on a PET-CT scan performed in November 2019. The regimen was changed to IV irinotecan (125 mg/m2) on days 1 and 8. In March 2020, PET-CT, which was performed after the 6th cycle of the third regimen, exhibited new multiple hypermetabolic lesions in the ribs, vertebral bodies, and the right iliac bone. The regimen was changed to FOL-FOX; unfortunately, the patient died of pneumonia in June 2020, 26 months after the second recurrence (Fig. 4).
Go to : 
Recurrence after a DFI of more than 10 years in GC is rare. Wu et al. [8] reported that 99.5% of recurrences were diagnosed within 7 years after curative resection for GC. Lee et al. [4] found that among 348 patients who developed recurrence, only 5 patients (1.4%) had a DFI of more than 10 years after gastrectomy. A previous study reported a case of recurrence involving the transverse colon 12 years after surgical resection [9]. These findings underscore that a recurrence after more than 10 years is rare in patients with GC. Notably, the recommended follow-up surveillance period is 5 years, according to the National Comprehensive Cancer Network guidelines [10]. The present case study demonstrates GC recurrence after the recommended surveillance period following curative resection. Therefore, physicians should be aware of the possibility of recurrence after the surveillance period.
The reported incidence rates of bone metastasis in GC in clinical practice are variable (0.9%–10%) [11-13]. However, bone metastasis with a DFI of more than 10 years is rare. Ahn et al. [11] reported that the median interval from surgery to the diagnosis of bone metastasis was 9 months (range: 0–73 months). Silvestris et al. [13] also reported similar results, with a range of 6.125–9.875 months and a median interval of 8 months, although their reported incidence rate was higher than that in other studies (10%). Kim et al. [14] reported that the incidence rate of bone marrow metastasis among patients who received palliative chemotherapy for metastatic, unresectable, or recurrent GC was 2.4% (39/1,598), and the median interval from the time of diagnosis of GC to the detection of bone marrow involvement was 161 days (range, 0–2,860 days). There are several case reports of GC metastasis to the bone and bone marrow with a long DFI [15-17]. However, to the best of our knowledge, in Korea, this was the first case of GC metastasis to the bone and bone marrow with a DFI of more than 10 years.
It is difficult to predict metastasis to the bone and bone marrow in patients with GC with a DFI of more than 10 years. Ahn et al. [11] reported that most patients with bone metastases had symptoms related to bone pain (88%) and elevated serum ALP levels (average, 484.4±785.6 U/L). According to Kim et al. [14], most patients with bone marrow metastases had bone involvement (79.5%) and underwent bone marrow biopsy due to thrombocytopenia (69.2%) and disseminated intravascular coagulopathy (20.6%). Common symptoms included bone pain (43.6%), active bleeding (20.6%), dyspnea (12.8%), elevated serum ALP (average 573 IU/L), and elevated serum lactate dehydrogenase (average 1,531 IU/L). The patient in this study had back pain, a non-specific symptom, and elevated ALP levels. These findings might have clinical importance. Non-specific symptoms such as back pain during the follow-up of patients with GC might be indicative of metastasis to the bone and bone marrow. This should prompt clinicians to conduct confirmatory tests.
There are two novel aspects of our study: first, metastasis to the bone and bone marrow in a patient with GC who had a DFI of more than 10 years is rare; second, the patient survived for approximately 26 months (779 days) after the diagnosis of bone marrow metastases. In the 39 cases reported by Kim et al. [14], the median survival time of all patients was 44 days (range, 2–252 days). In the report published by Kwon et al. [18], the median survival time of all patients was 37 days (range, 12.5–61.5 days). The median survival time of eight patients in the study by Ergun et al. [19] was 72 days (range, 3–145 days). The authors of the aforementioned studies analyzed several adverse prognostic factors such as supportive care without chemotherapy, low serum sodium level (<133 mmol/L), presence of lung metastasis, presence of peritoneal seeding, and elevated alanine aminotransferase level (ALT, >31 IU/L). In this case, the patient had no adverse prognostic factors, and serum sodium levels (139 mEq/L) and ALT levels (16 IU/L) were both within the normal range (normal serum sodium range, 135–145 mEq/L; normal ALT range, 0–40 IU/L). Palliative chemotherapy might have influenced the duration of survival in this patient. However, chemotherapy regimens for bone and bone marrow metastasis in GC have not been adequately optimized due to a lack of prospective studies. Further analysis is necessary to establish a therapeutic strategy for bone marrow metastasis of GC.
In conclusion, metastasis to the bone and bone marrow in GC after a DFI of more than 10 years is extremely rare. This patient had an unusually long duration of survival compared to that reported in previous studies showing a median survival time of less than 3 months. To the best of our knowledge, this is the first case of metastasis of the gastric SRCC to the bone and bone marrow in Korea, after a DFI of more than 10 years. We also present a photographic image of the bone marrow aspirate smear.
Go to : 
REFERENCES
1. Torre LA, Siegel RL, Ward EM, Jemal A. 2016; Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomarkers Prev. 25:16–27. DOI: 10.1158/1055-9965.EPI-15-0578. PMID: 26667886.

2. Jung KW, Won YJ, Kong HJ, Lee ES. 2019; Prediction of cancer incidence and mortality in Korea, 2019. Cancer Res Treat. 51:431–7. DOI: 10.4143/crt.2019.139. PMID: 30913864. PMCID: PMC6473283.

3. Kim CD, Chang MC, Roh HR, Chae GB, Yang DH, Choi WJ. 2003; Factors influencing recurrence after curative resection for advanced gastric cancer. J Korean Surg Soc. 65:301–8.
4. Lee JH, Kim HI, Kim MG, Ha TK, Jung MS, Kwon SJ. 2016; Recurrence of gastric cancer in patients who are disease-free for more than 5 years after primary resection. Surgery. 159:1090–8. DOI: 10.1016/j.surg.2015.11.002. PMID: 26747230.
5. Yoo CH, Noh S, Shin DW, Choi SH, Min JS. 2000; Recurrence following curative resection for gastric carcinoma. Br J Surg. 87:236–42. DOI: 10.1046/j.1365-2168.2000.01360.x. PMID: 10671934.

6. American Joint Committee on Cancer. 1992. Manual for staging of cancer. 4th ed. JB Lippincott;Philadelphia:
7. American Joint Committee on Cancer. 1997. AJCC cancer staging manual. 5th ed. Lippincott Williams - Raven;Philadelphia:
8. Wu CW, Lo SS, Shen KH, Hsieh MC, Chen JH, Chiang JH, et al. 2003; Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. World J Surg. 27:153–8. DOI: 10.1007/s00268-002-6279-7. PMID: 12616428.

9. Ku KH, Park SJ, Kim JH, Kwon HJ, Chang HK, Park JG. 2017; Gastric cancer recurrence in 12 years after surgical resection. Korean J Gastroenterol. 70:296–300. DOI: 10.4166/kjg.2017.70.6.296. PMID: 29277092.

10. National Comprehensive Cancer Network. 2019. Gastric cancer. Version 2. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Last accessed on Mar 2019.
11. Ahn JB, Ha TK, Kwon SJ. 2011; Bone metastasis in gastric cancer patients. J Gastric Cancer. 11:38–45. DOI: 10.5230/jgc.2011.11.1.38. PMID: 22076200. PMCID: PMC3204476.

12. Turkoz FP, Solak M, Kilickap S, Ulas A, Esbah O, Oksuzoglu B, et al. 2014; Bone metastasis from gastric cancer: the incidence, clinicopathological features, and influence on survival. J Gastric Cancer. 14:164–72. DOI: 10.5230/jgc.2014.14.3.164. PMID: 25328761. PMCID: PMC4199883.

13. Silvestris N, Pantano F, Ibrahim T, Gamucci T, De Vita F, Di Palma T, et al. 2013; Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey. PLoS One. 8:e74402. DOI: 10.1371/journal.pone.0074402. PMID: 24204569. PMCID: PMC3810275.

14. Kim HS, Yi SY, Jun HJ, Lee J, Park JO, Park YS, et al. 2007; Clinical outcome of gastric cancer patients with bone marrow metastases. Oncology. 73:192–7. DOI: 10.1159/000127386. PMID: 18418012.

15. Blanchette P, Lipton JH, Barth D, Mackay H. 2013; Case report of very late gastric cancer recurrence. Curr Oncol. 20:e161–4. DOI: 10.3747/co.20.1200. PMID: 23559883. PMCID: PMC3615867.

16. Noda N, Sano T, Shirao K, Ono H, Katai H, Sasako M, et al. 1996; A case of bone marrow recurrence from gastric carcinoma after a nine-year disease-free interval. Jpn J Clin Oncol. 26:472–5. DOI: 10.1093/oxfordjournals.jjco.a023267. PMID: 9001355.

17. Mizuno I, Izeki O, Nakahara S, Kawamoto K, Onga T, Matsuoka H, et al. 1998; Disseminated carcinomatosis of the bone marrow occurring 11 years after subtotal gastrectomy for gastric cancer. Rinsho Ketsueki. 39:670–5. PMID: 9796401.
18. Kwon JY, Yun J, Kim HJ, Kim KH, Kim SH, Lee SC, et al. 2011; Clinical outcome of gastric cancer patients with bone marrow metastases. Cancer Res Treat. 43:244–9. DOI: 10.4143/crt.2011.43.4.244. PMID: 22247710. PMCID: PMC3253867.

19. Ergun Y, Uncu D, Yazini O, Ucar G, Konca Karabuga E, Zengin N, et al. 2017; Gastric cancer patients with bone marrow metastasis: a single-center experience and review of the literature. EJMO. 1:160–3. DOI: 10.14744/ejmo.2017.65365.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download