This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Analysis of body fluids aids in diagnosis and monitoring disease. However, only a few testing platforms and reagents have been validated for a range of body fluids or analytes. In this study, we evaluated a testing system, which has been approved for blood samples, in analyzing body fluid specimens upon matrix mixing.
Methods
Serum and body fluid samples, including cerebrospinal fluid (CSF), ascites, pleural fluid, amniotic fluid, and synovial fluid, were mixed, then the matrix effect and linearity for major analytes, namely amylase, chloride, glucose, LDH, and protein were evaluated (N=30 serum-body fluid pairs) on the Cobas 8000 c702. The obtained data was compared with that of open reagents evaluated on the Architect c16000.
Results
For all analyte-body fluid pairs, the mean percent recovery ranged from 98.4% to 101.7%, and this was within the acceptable range for matrix effect. In the linearity test, maximum non-linearity for each analyte-body fluid pair ranged from -5.0% to +4.2%. In interference test, proteins showed positive hemolytic, icteric, and lipemic interference in CSF and hemolytic interference in amniotic fluid. There was no significant interference in the other analyte-body fluid pairs. Results were highly correlated between the Cobas 8000 c702 and the Architect c16000 system.
Conclusions
Our findings revealed that the matrix effect of major analytes in body fluid specimens can be excluded and they also validated the linearity of the analytes in the body fluid specimens. Therefore, reagents specified for blood samples can be readily adopted for the analysis of body fluids.
초록
배경
체액 검체는 질병의 진단과 관찰에 유용하게 활용될 수 있으나 혈액 외의 검체를 대상으로 성능이 검증된 분석장비와 시약은제한적이다. 본 연구에서는 혈액 검체를 승인된 장비와 시약을 대상으로 체액 검체에 대한 적용가능성을 확인하였다.
방법
혈청 검체와 뇌척수액, 복수, 흉막액, 양수, 관절낭액의 5종체액 검체를 다양한 비율로 혼합하여 혈청-특정 체액 검체 조합당 30개씩 총 150개의 혼합물을 제조하였고 주요 분석물질을 대상으로 기질효과와 직선성을 평가하였다. 본 평가는 Cobas 8000 c702 자동분석장비와 전용 시약을 사용하여 시행하였고, 타장비와 범용 시약을 사용하여 측정한 결과와 비교하여 상관성을 평가하였다.
결과
기질효과 평가에서는 모든 분석물질-체액 검체의 조합에서평균 회수율의 분포는 98.4-101.7%로 허용범위 이내에 있었으며,직선성 평가에서의 최대 비직선성은 -5.0-4.2%로 허용범위 내에 존재하였다. 간섭효과의 평가에서는 단백질 농도측정에 한해 뇌척수액에서는 혈색소, 빌리루빈, 지질에 의한, 양수에서는 혈색소에 의한 유의한 수준의 간섭이 관찰되었다. Roche Cobas 800 c702장비와 전용 시약을 사용하여 시행한 본 평가의 측정값은 Abbott Ar-chitect c16000 장비에서 범용 시약을 사용하여 측정한 값과 높은 상관성을 보였다.
결론
본 연구의 결과로 보아 체액 검체와 혈액 검체의 기질효과 간에 유의한 차이를 배제할 수 있으며, 평가를 시행한 농도범위 내에서의 직선성이 확인되었으므로 체액 검체의 검사에 혈액 검체를 대상으로 검증된 검사 장비와 시약을 사용하는 전략을 고려할 수 있다.
Keywords: Body fluid chemistry, Matrix effect, Test performance evaluation
INTRODUCTION
Laboratory tests play a major role in clinical decision making during disease screening, diagnosis, and therapeutic response svgmonitoring [
1,
2]. Therefore, laboratory tests should be validated for accurate and reliable results. Many manufacturers provide laboratory test platforms, consisting of the analyzer and reagents, along with certain performance specifications. However, these specifications are usually for blood-derived specimens, such as serum or plasma, and not for all types of body fluids. Even though laboratory tests for specific body fiuid specimens are requested for clinical needs [
3], and the test is occasionally validated for this body fiuid specimen, these validations are not routinely performed. Consequently, the performances of laboratory tests for analysis of body fiuids are not FDA-approved [
4-
6] and cannot be warranted.
The Clinical and Laboratory Standards Institute (CLSI) guideline C49-A describes that the “matrix” surrounding the measurand of interest can alter the physicochemical property of the measurand and affect the test results. It also provides guidelines for the validation of body fiuid chemistry tests, whose performance specifications have not yet been approved [
7].
In this study, we evaluated the performance of a test system that determines the levels of certain analytes in body fiuid specimens using specifications that have been validated for blood-derived samples. We specifically focused on the evaluation of matrix effect and verified the application of the test system to the specimens that are not serum or plasma.
MATERIALS AND METHODS
1. Specimens and analyzers
Analytes evaluated in this study were selected on the basis of their established clinical utility and demand [
3], and consisted of the following: amylase for diagnosing and monitoring pancreatic disease; chloride (Cl) for diagnosing cystic fibrosis; glucose, lactate dehydrogenase (LDH), and total protein for distinguishing the origin of abnormal pleural fiuid or ascites, and diagnosing infection. Body fiuid specimens evaluated in the present study consisted of the following: cerebrospinal fiuid (CSF), ascites, pleural fluid, amniotic fluid, and synovial fluid. These specimens obtained were residual samples that were left after completion of clinical tests. For each analyte, matrix effect, linearity, analytical measurement range (AMR), and interference were evaluated on the Cobas 8000 c702 analyzer (Roche Laboratories, Basel, Switzerland) using specific reagents (Roche) designed for the closed system and specifications that have been validated for blood-derived samples. Method comparison was performed by comparing the results obtained from the aforementioned analyzer and with that of the Architect c16000 analyzer (Abbott Laboratories, IL, USA) using open reagents (amylase, LQDIA AMY Asan; Cl, DENKA Ion charge; glucose, SICDIA L GLU REAGENT; LDH, SEKISUI AUTO ANALYZER LDH-L; protein, SICDIA TP reagent), which are used for routine laboratory tests. All the tests were validated for blood-derived samples by the manufacturers.
2. Study design
1) Matrix effect
The matrix effect of body fiuid was evaluated in mixed samples of serum and body fiuid specimens using a modified protocol of a previous study by Swift et al. [
8]. According to the CLSI C49-A guideline, ten specimens of each type of body fiuid should be used to minimize sample-specific variation. Therefore, 50 body fiuid specimens (ten specimens per body fiuid type) were prepared. These specimens were paired with 50 serum specimens, and then the five aforementioned analytes, namely amylase, Cl, glucose, LDH, and protein, were measured on the Cobas 8000 c702 analyzer in serum mode. The test samples were prepared from the 50 serum-body fiuid pairs in three ratios, 3:1, 2:2, and 1:3 of body fiuid to serum. Thus, a total of 150 mixed samples, consisting of 30 mixed samples per serum-body fiuid pair, with various mixing ratios were subjected to testing, and the obtained values for each analyte were compared to the “expected” values, which were values obtained from pure serum and pure body fiuid samples prior to mixing. As described in a previous study [
4], the percent recovery for each sample was calculated as follows and presented on scatter plot:
Percent recovery = (measured value/expected value) * 100%
For each type of body fiuid, %recovery values for each analyte were obtained, and then the mean and 95% confidence intervals (CI) were calculated. An average percent recovery within the range of analyte-specific desirable total error, as suggested by Westgard [
9], was regarded as “acceptable”.
2) Linearity and AMR
Body fluid specimens with low levels of amylase, glucose, LDH, protein and a high level of Cl (except synovial fiuid which commonly has higher LDH than serum) were mixed with serum samples that had a near-AMR upper limit concentration of each analyte in ratios 3:1, 2:2, 1:3, and 1:7 of body fiuid to serum. The linearity of body fiuids was assessed according to CLSI EP06-A guideline [
10], wherein expected values were plotted on the x-axis and measured values on the y-axis. The linearity of analyte in the body fiuid specimen was considered acceptable when the regression for six pairs of values showed best fit for linear regression or best fit for polynomial regression with a percent difference smaller than 10% compared to the linear regression of the tested concentration range.
3) Interference
Serum samples with high concentration of interference components, such as hemoglobin (H-index), bilirubin (I-index), triglyceride (L-index), within the manufacturer-claimed acceptable limit in specification, and serum and body fiuid samples with “low index” were prepared for interference evaluation. Each sample was measured for five test items, and the high index serum samples were serially diluted, 2-, 4-, 8-, and 16-fold with low index serum or body fiuid as diluent. Finally, 600 samples for the five analytes (5), the five types of body fiuid with low index and their corresponding five low index serum samples as diluent (10), and the three types of interference material with four-fold serial dilutions (12) were prepared. For each analyte and body fiuid type, the measured values were obtained, and then the percent recovery was determined using calculated expected values. The effect of interference material in serum and body fiuids was regarded to be equivalent if the percent recovery of serum- and body fiuid-diluted samples were not significantly different. When the percent recovery of body fiuid diluent was significantly different from that of serum, it was compared with the results of the matrix effect experiment performed without interference material, to determine whether the difference has originated due to the presence of an interference material or an intrinsic matrix effect. Comparison with biological variation was performed to evaluate whether the difference originating from interference is clinically significant or not.
4) Correlation
Five test items in 20 specimens for each body fiuid type were measured using the Cobas 8000 c702 analyzer with its specific reagent and the Architect c16000 analyzer with an open system reagent. For method comparison, the results obtained from the two test systems were compared by Deming regression, and the slope, intercept, and their 95% CIs, coefficients of determination (R2) were calculated. Comparisons with a slope of 1 and an intercept of 0 in the 95% CI were regarded as “equivalent”, and R2 larger than 0.95 was considered to be “transferable” even if it did not meet the aforementioned conditions.
3. Statistical analysis
Statistical analysis of the matrix effect evaluation and interference test was performed with IBM SPSS Statistics for Windows Version 25.0 (IBM Corp., Armonk, NY, USA). The EP Evaluator 12 (Data Innovations, Burlington, VT, USA) was used for the linearity test and method comparison, and MedCalc version 14.8.1 (MedCalc Software, Ostend, Belgium) was used for the interference test.
RESULTS
1. Matrix Effect
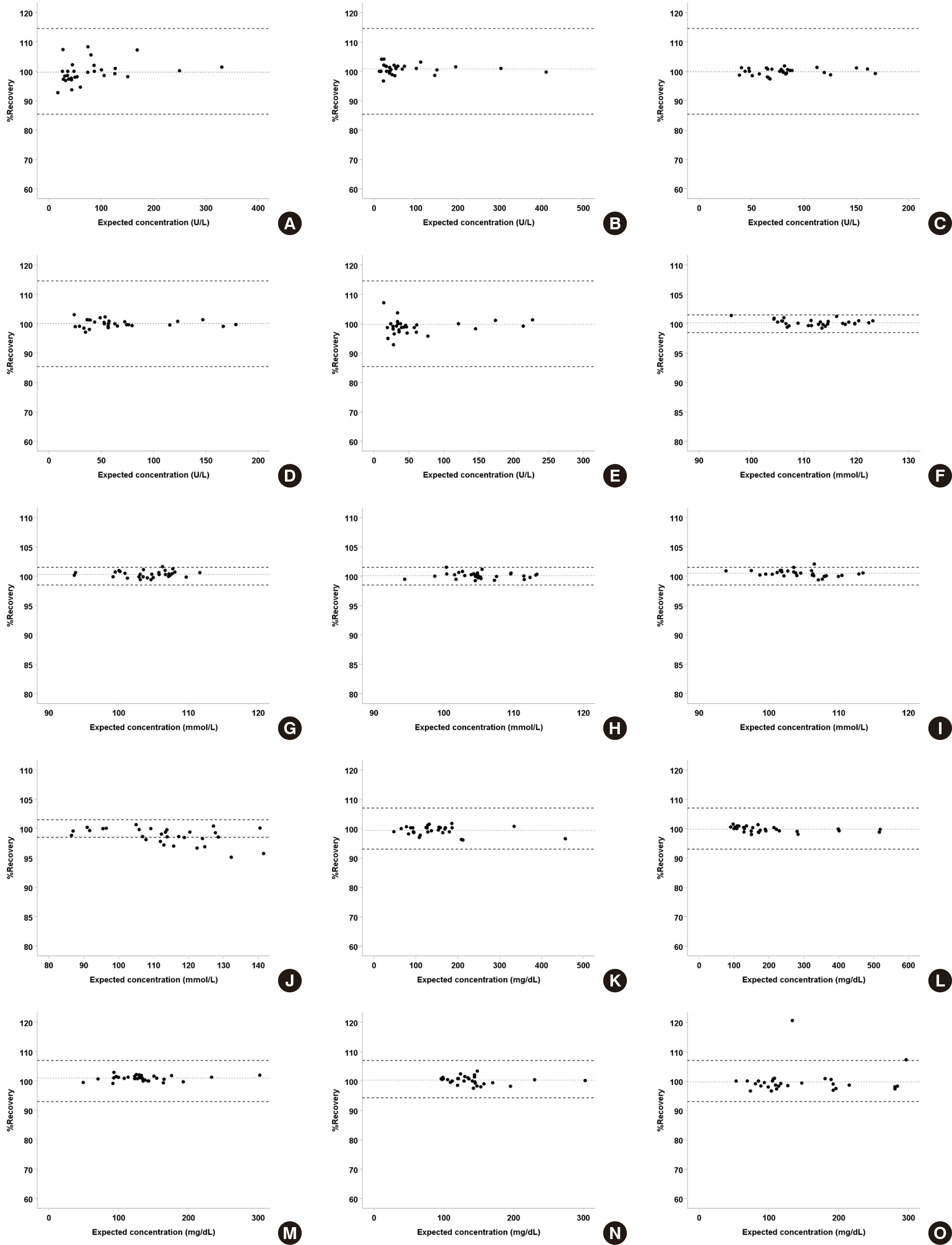
Thirty pairs of analyte-body fiuid specimens were analyzed, and the percent recovery, percent difference, and absolute percent difference of each test sample was calculated to determine the mean values and 95% CIs. The mean percent recovery ranged from 98.4% (protein-CSF) to 101.7% (LDH-pleural fiuid), and the lower and upper limit of the 95% CI ranged from 96.9% (protein-CSF) to 102.8% (LDH-pleural fiuid) (
Table 1). Mean percent recovery of a few analyte-body fiuid pairs were significant with 100% recovery. However, the differences were within range of allowable variation (
Fig. 1).
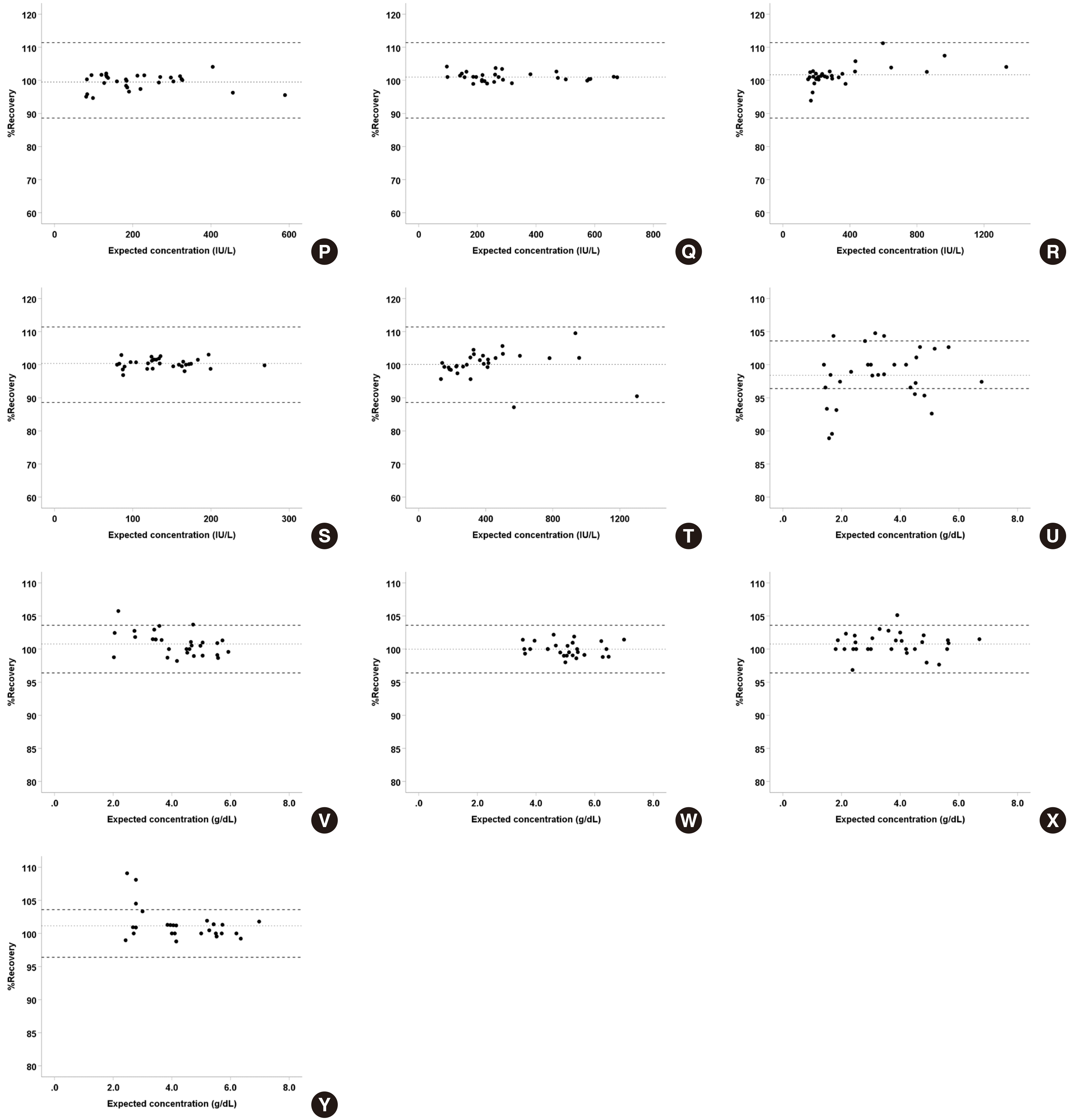
2. Linearity and AMR
For the evaluation of linearity, most analyte-body fiuid pairs showed best fit for linear regression, and only some of them showed best fit for polynomial regression and had a non-linearity of less than 10% compared to the linear fit. The recovery ranged from 89.2-110.0% (
Table 2).
3. Interference
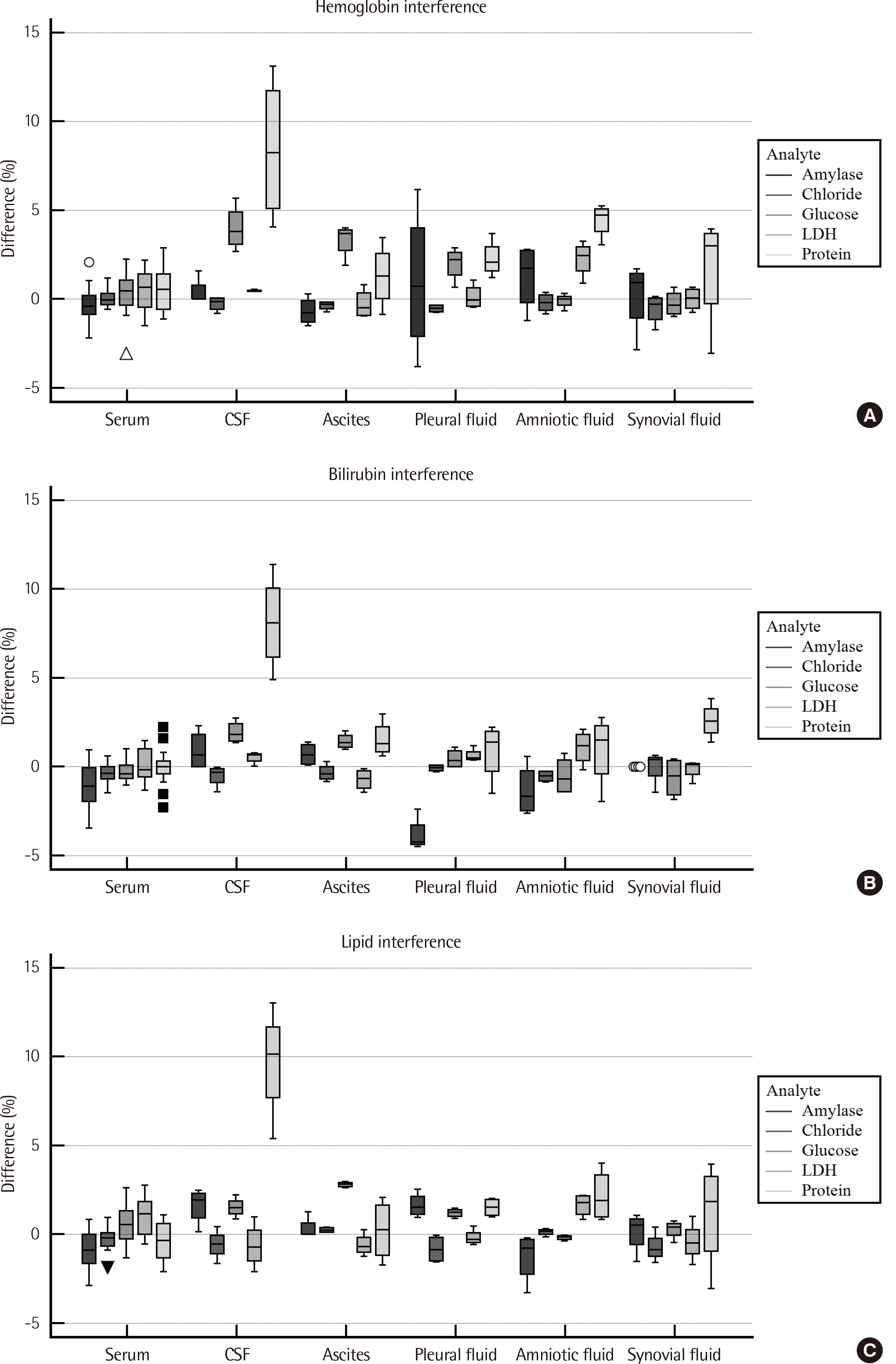
Serum samples that showed high H-index ranging from 135 to 216, and I-index ranging 31–36, corresponding to the presence of hemoglobin or bilirubin, respectively, in the order of mg/dL, and L-index ranging 89-153, corresponding to the presence of artificial lipid (Intralipid; Kabi-Pharmacia) in the order of mg/dL, were designated as “high-index” samples. “Low-index” serum and body fiuid samples had very low, nearly zero interference index. The results of interference evaluation performed by diluting high-index serum with low-index serum and body fiuid are shown in
Table 3. When the recovery of body fiuid diluent for specific analytes paired with interference material was compared with that of serum diluent using the Mann–Whitney U test, most body fiuids, except CSF and amniotic fiuid, showed a statistical equivalence with
P-value >0.05 or analytical equivalence with mean percent difference smaller than Westgard’s allowable error. For protein measurements, CSF showed positive interference compared to low-index serum diluent for hemoglobin, bilirubin and lipid, whereas amniotic fiuid showed positive interference for hemoglobin only (
Fig. 2).
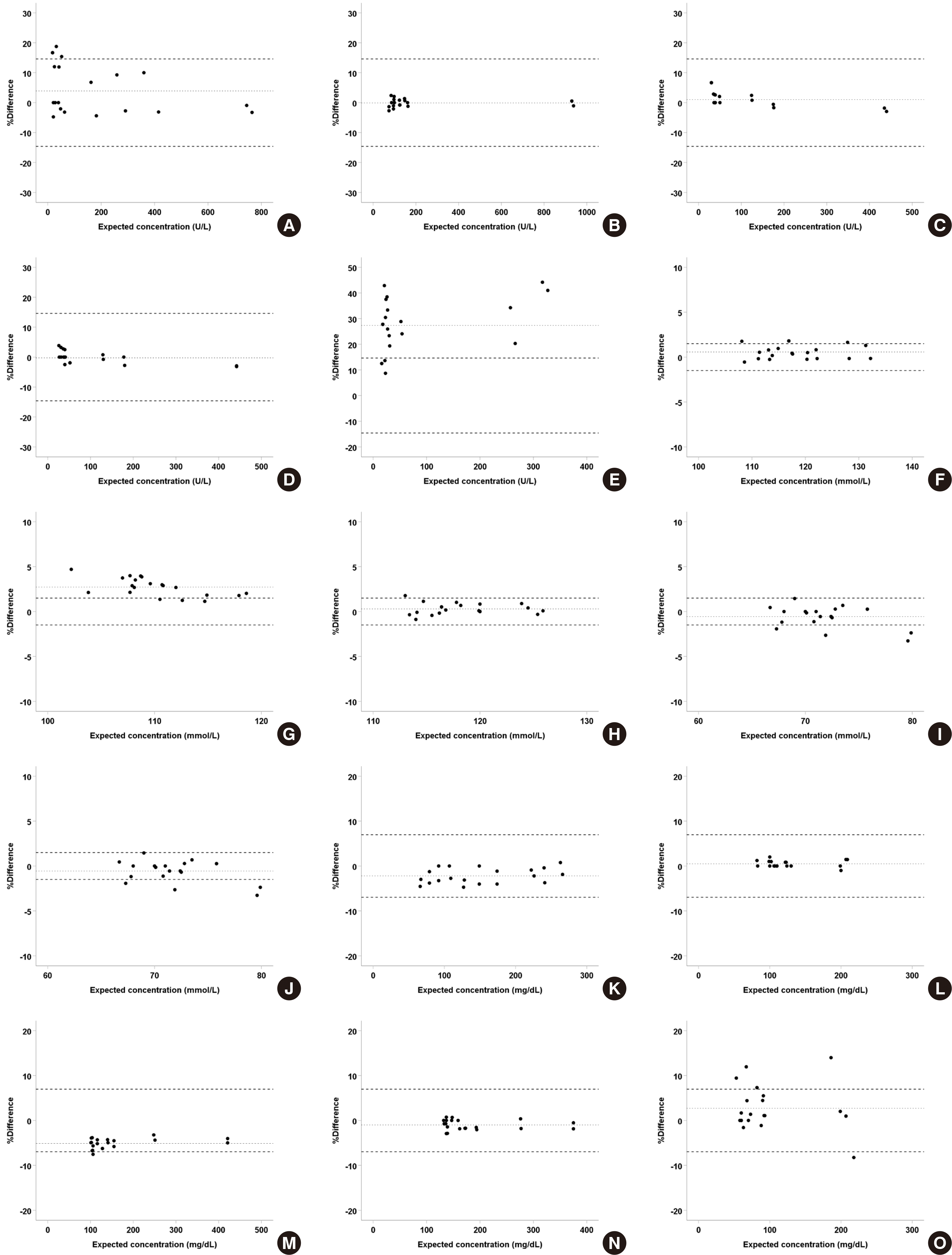
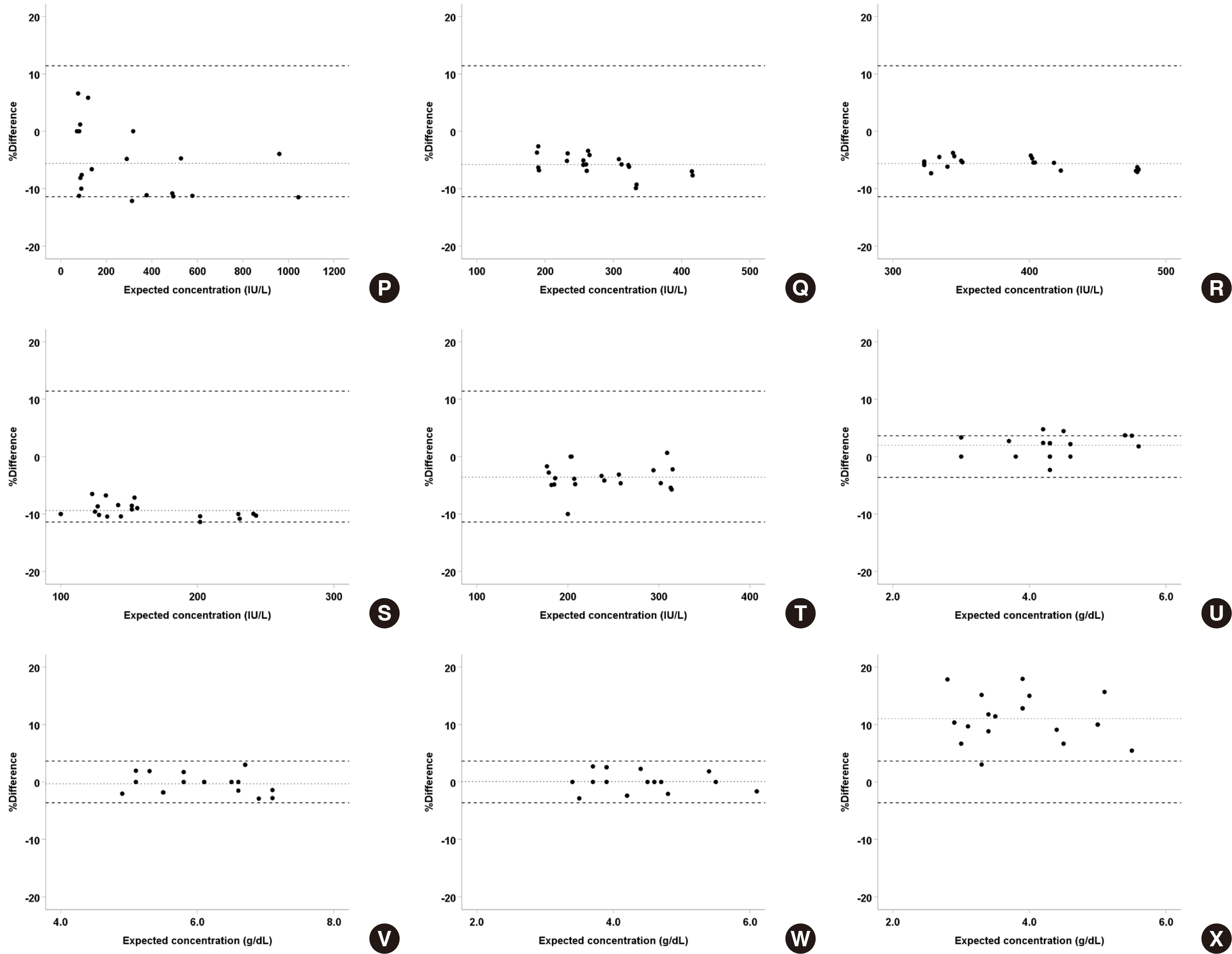
4. Correlation
Data obtained from Cobas 8000 c702 (Roche) with its specific reagent were compared with those from Architect c16000 (Abbott) with an open system reagent using Deming regression. It was found that many analyte-body fiuid specimen pairs showed statistical equivalence between both platforms with a slope of 1 and intercept of 0 in the 95% CI. Analytes that were not equivalent showed high correlation between two systems with R
2 > 0.95 except for Cl in ascites (
Table 4). When the percent differences between two platforms were presented as a Bland–Altman plot, most tests showed a mean percent difference smaller than Westgard’s allowable variation, and some tests like amylase and protein in synovial fiuid were transferable; only Cl in ascites had a nonequivalent result, similar to that of the regression analysis (
Fig. 3). Therefore, values obtained from these systems can be interconverted using an appropriate transformation equation, as they are statistically equivalent.
DISCUSSION
In the present study, the performance of a testing system, which is conventionally used of blood-derived specimens, for the analysis of body fiuid specimens was validated. The matrix effect, linearity, AMR, effect of interference material, and method comparison were assessed for five analytes (amylase, Cl, glucose, LDH, protein) in five body fiuid specimen types (CSF, ascites, pleural fiuid, amniotic fiuid, synovial fiuid).
We found that the matrix effect showed a percent difference and an absolute percent difference smaller than 5% for all the analyte-body fiuid pairs, and this was within the acceptable level of matrix effect in body fiuid samples compared to that of blood-derived samples. Therefore, matrix interference can be reasonably excluded, and the test specifications that have been validated for blood samples can be adopted for the analysis of body fiuid samples, according to the guidance of College of American Pathologists [
11]. For the test of linearity, body fiuid specimens exhibited non-linearity within an acceptable range of 5%. Positive interference was demonstrated for the measurement of protein in body fiuid specimens, particularly for all kinds of interference material in CSF and for hemoglobin in amniotic fiuid. Therefore, CSF and amniotic fiuid are more vulnerable to interference than serum specimens are, and therefore, reagents approved for these specimens are recommended, else special attention should be paid during the interpretation of results if approved reagents are unavailable. For most of the analyte-body fiuid pairs, comparison test showed statistically equivalent or highly correlative results (R
2 >0.95) between the two examined test platforms, that is the Cobas 8000 c702 analyzer and the Architect c16000 analyzer, except for the analysis of Cl in ascites. Thus, the results obtained from these two platforms can be interconverted.
This study has certain limitations, including the mixing ratios used and the small sample size of body fiuid specimens. First, the CLSI EP06-A guideline [
10] recommends that the added solution should not exceed 10% of the final volume during sample preparation, and therefore most samples evaluated in the present study were more appropriate for comparisons with serum, rather than for the evaluation of matrix effect of body fiuid specimens. Additionally, although the equivalence of matrix effect between serum and other body fiuids was reported in the present evaluation, the range of concentrations of analytes was not wide enough to cover the entire AMR for the serum samples. Therefore, the matrix effect equivalence was confirmed only for limited range of concentrations, which also affects the results of other tests conducted in this study. The limited number of samples included in this study was not large enough to secure enough statistical power, except for the evaluation of matrix effect, which contained 30 values for each analyte-body fiuid pairs. Thus, the results of this study, except for that of the matrix effect, can be used only to support, but not demonstrate, the use of specifications for blood samples in the analysis of body fiuid specimens. It might be worth increasing the number of specimens in future performance evaluations of test systems for the analysis of body fiuid specimens. In addition to this limitation of study design, the intrinsic drawbacks of body fiuid analysis include issues associated with sample collection and handling, viscosity, lack of reliable reference intervals, and heterogeneity of fiuid samples in various disease conditions [
12]. Further studies are required to address these drawbacks.
In spite of some limitations, this study has demonstrated that matrix interference can be reasonably excluded during the analysis of body fiuids using commonly used assays, and analyzing systems and reagents that have been approved for blood specimens can be used for the analysis of body fiuids, as well. Although we evaluated a specific analyzer and reagent, we expect that our findings can be applied to other platforms through appropriate method comparison. Our study is helpful to laboratories that wish to adopt method specifications for blood samples to body fiuid tests or for studies aimed to validate body fiuid tests.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download