Dear Editor,
Primary bone marrow lymphoma (PBML) is a rare form of lymphoma that originally arises in the bone marrow. The criteria for diagnosis of PBML include (a) pathological evidence of isolated bone marrow (BM) infiltration regardless of peripheral blood involvement, (b) absence of nodal or extranodal involvement, (c) no evidence of tumor formation, (d) no evidence of bony trabecular destruction on BM biopsy, and (e) exclusion of leukemia/lymphoma that involves the BM such as chronic lymphocytic leukemia (CLL)/small lymphocytic leukemia. The majority of PBMLs are confirmed to be diffuse large B-cell lymphomas (DLBCLs), with rare cases of other types of B-cell non-Hodgkin lymphomas (B-NHLs) reported as PBML [1]. The co-existence of PBML and other hematopoietic disorders has not been reported to date. We report a patient diagnosed as having PBML/B-NHL and essential thrombocythemia (ET) concomitantly. The Institutional Review Board of Yongin Severance Hospital, Yongin, Korea, approved this study (IRB No. 9-2021-0005) and waived the need for informed consent.
A 72-year-old female with no specific medical history visited an outpatient clinic at Yongin Severance Hospital in August 2020 with a five-year history of intermittent palpitation. She exhibited leukocytosis and thrombocytosis with a white blood cell (WBC) count of 22.87×109/L (42% neutrophils, 51% lymphocytes, 5% monocytes, 2% eosinophils), hemoglobin of 132 g/L, and platelet count of 1,260×109/L in the complete blood count (CBC). Approximately 17% atypical lymphocytes, which had abundant cytoplasm and occasionally showed a cleaved nucleus, were observed on a peripheral blood smear (Fig. 1A, B). Peripheral blood flow cytometry (Beckman Coulter, Miami, FL, USA) revealed a monoclonal B-cell population with kappa light chain restriction. These cells were strongly positive for CD19, CD20, CD79b, and surface immunoglobulin (sIg) kappa light chain, and negative for CD5, CD10, FMC7, CD23, CD38, CD138, and sIg lambda light chain. This monoclonal B-cell population accounted for 62% of the total lymphocytes. Although BM aspiration was dry-tapped, some atypical lymphocytes, which were larger than the surrounding small lymphocytes and occasionally had an indented or cleaved nucleus, were observed on the BM aspirate smear (Fig. 1C, D). BM biopsy showed marked megakaryocytic hyperplasia, in which abnormal large and giant megakaryocytes with hypersegmented nuclei were occasionally observed (Fig. 1E). Multifocal non-paratrabecular and paratrabecular infiltrates of small to medium-sized lymphocytes were observed, which were immunohistochemically positive for BCL2 and CD20, and negative for BCL6, cyclin D1, ZAP-70, and CD138 (Fig. 1F). The atypical lymphocytes were focally positive for CD5 and CD23.
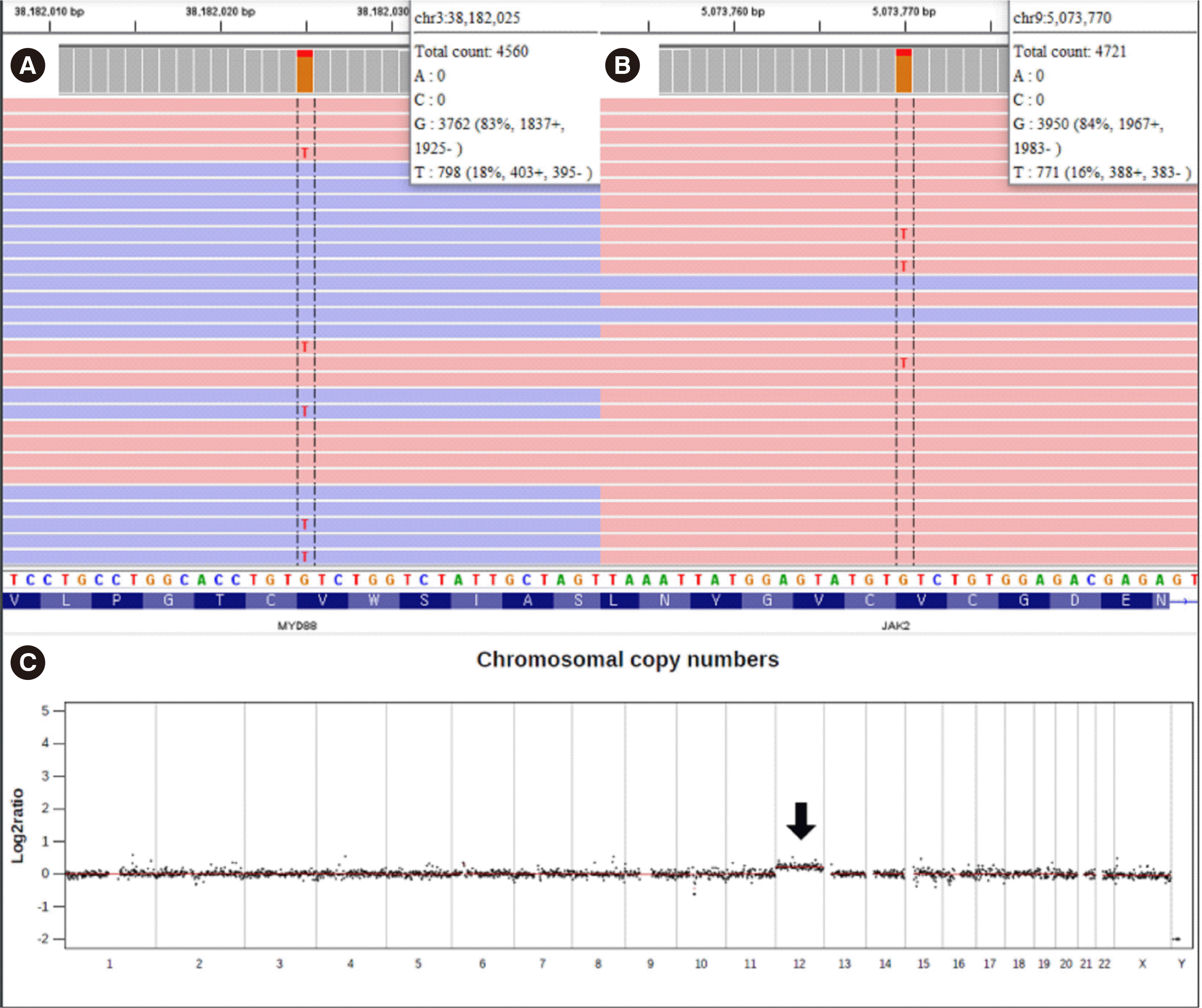
Clonality analysis, next-generation sequencing (NGS), karyotype analysis, and FISH were performed on the available BM aspirate. Clonal rearrangements in Ig heavy chain variable 3 family (IGHV3-23; 64.8% of total reads) and Ig kappa light chain variable 3 family (IGKV3D-20; 48.1% of total reads) were demonstrated using LymphoTrack (Invivoscribe Technologies, San Diego, CA, USA). NGS was performed using the NextSeq 550Dx platform (Illumina, San Diego, CA, USA) and a custom panel targeting 497 genes associated with hematopoietic disorders; somatic variants of MYD88 p.Val217Phe (17.5% variant allele frequency [VAF]) and JAK2 p.Val617Phe (16.3% VAF) were detected (Fig. 2A, B). Trisomy 12 was suspected from copy number analysis using NGS data (Fig. 2C) and confirmed using G-banding: 47,XX,+12[4]/46,XX [16]. The t(14;18)(q32;q21) was not detected by FISH. No paraprotein was detected in protein electrophoresis. There was no definite evidence of lymph node enlargement or splenomegaly in computed tomography scans of the neck, chest, abdomen, and pelvis. No discernible fluorodeoxyglucose uptake suggesting malignant processes was observed in positron emission tomography-computed tomography.
Given these ambiguous findings, several B-NHLs needed to be discriminated. There was more support for the possibility of atypical CLL or non-CLL lymphoma than for classical CLL based on the negative staining for CD5 and CD23 and detection of trisomy 12. Morphologically aberrant lymphocytes and prolymphocytic cells are more frequent in atypical CLL with trisomy 12 [2]. However, CD5- and CD23-negative atypical CLL are very rare, and trisomy 12 is found in other lymphomas, such as mantle cell lymphoma and late-stage follicular lymphoma (FL) [2-4]. Among the several reported cases of a concomitant diagnosis of CLL and myeloproliferative neoplasm (MPN), none was a definite atypical CLL [5]. The possibility of high-grade FL was assessed since CD10 and BCL6 tend to diminish as the FL grade increases [6]. Similarly, t(14;18)(q32;q21) is infrequent in grade-3 FL [7]. Leukemic manifestation in PBML with the FL histological subtype is common [1]. More than 90% of lymphoplasmacytic lymphomas have MYD88 variants, most of which are p.Leu265Pro; however, p.Val217Phe was detected in this case. Owing to its low prevalence, the biological role of the MYD88 p.Val217Phe variant has not been clarified. However, it may contribute to lymphomagenesis related to nuclear factor kappa B activation, considering its recurrent nature and location within the MyD88 Toll/interleukin-1 receptor domain [8]. DLBCL was morphologically ruled out [3].
A watch-and-wait strategy was applied as an FL-oriented treatment, and oral hydroxyurea and aspirin were administered for ET. Ten months after the diagnosis, the patient did not show any symptoms, and the CBC indicated a WBC count of 12.43×109/L (79% lymphocytes), hemoglobin of 98 g/L, and platelet count of 308×109/L. Although a poor prognosis is generally predicted in PBML patients, the prognosis is significantly dependent on the histological subtype, which can explain the clinical outcome of our patient [1].
Patients with MPN have an increased risk of lymphomas. Although the relationship is uncertain, there may be shared pathogenic mechanisms occurring in early events of the hematopoietic process that increase the risk of both MPN and lymphoma [9]. Progressively increased genomic instability in MPN can lead to other lymphoma-related genetic variants [10].
In conclusion, we report the first case of a concomitant diagnosis of PBML and MPN. Although the type of B-NHL was never specified and a diagnosis of PBML cannot be confirmed if the B-NHL is a lymphoma involving the BM (such as atypical CLL), this case shows that a comprehensive evaluation including molecular diagnostic tools can play a more important role in the diagnosis of lymphoma with unusual features and co-existing hematopoietic disorders.
Notes
REFERENCES
1. Martinez A, Ponzoni M, Agostinelli C, Hebeda KM, Matutes E, Peccatori J, et al. 2012; Primary bone marrow lymphoma: an uncommon extranodal presentation of aggressive non-Hodgkin lymphomas. Am J Surg Pathol. 36:296–304. DOI: 10.1097/PAS.0b013e31823ea106. PMID: 22251943.
2. Frater JL, McCarron KF, Hammel JP, Shapiro JL, Miller ML, Tubbs RR, et al. 2001; Typical and atypical chronic lymphocytic leukemia differ clinically and immunophenotypically. Am J Clin Pathol. 116:655–64. DOI: 10.1309/7Q1J-1AA8-DU4Q-PVLQ. PMID: 11710681.

3. Onaindia A, Medeiros LJ, Patel KP. 2017; Clinical utility of recently identified diagnostic, prognostic, and predictive molecular biomarkers in mature B-cell neoplasms. Mod Pathol. 30:1338–66. DOI: 10.1038/modpathol.2017.58. PMID: 28664939.

4. Mohamed AN, Palutke M, Eisenberg L, Al-Katib A. 2001; Chromosomal analyses of 52 cases of follicular lymphoma with t(14;18), including blastic/blastoid variant. Cancer Genet Cytogenet. 126:45–51. DOI: 10.1016/S0165-4608(00)00383-6. PMID: 11343778.

5. Todisco G, Manshouri T, Verstovsek S, Masarova L, Pierce SA, Keating MJ, et al. 2016; Chronic lymphocytic leukemia and myeloproliferative neoplasms concurrently diagnosed: clinical and biological characteristics. Leuk Lymphoma. 57:1054–9. DOI: 10.3109/10428194.2015.1092527. PMID: 26402369. PMCID: PMC4837105.

6. Horn H, Schmelter C, Leich E, Salaverria I, Katzenberger T, Ott MM, et al. 2011; Follicular lymphoma grade 3B is a distinct neoplasm according to cytogenetic and immunohistochemical profiles. Haematologica. 96:1327–34. DOI: 10.3324/haematol.2011.042531. PMID: 21659362. PMCID: PMC3166103.

7. Godon A, Moreau A, Talmant P, Baranger-Papot L, Geneviève F, Milpied N, et al. 2003; Is t(14;18)(q32;q21) a constant finding in follicular lymphoma? An interphase FISH study on 63 patients. Leukemia. 17:255–9. DOI: 10.1038/sj.leu.2402739. PMID: 12529690.

8. Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, et al. 2011; Oncogenically active MYD88 mutations in human lymphoma. Nature. 470:115–9. DOI: 10.1038/nature09671. PMID: 21179087. PMCID: PMC5024568.

9. Holst JM, Plesner TL, Pdersen MB, Frederiksen H, Møller MB, Clausen MR, et al. 2020; Myeloproliferative and lymphoproliferative malignancies occurring in the same patient: a nationwide discovery cohort. Haematologica. 105:2432–9. DOI: 10.3324/haematol.2019.225839. PMID: 33054083. PMCID: PMC7556673.
10. Vannucchi AM, Masala G, Antonioli E, Chiara Susini M, Guglielmelli P, Pieri L, et al. 2009; Increased risk of lymphoid neoplasms in patients with Philadelphia chromosome-negative myeloproliferative neoplasms. Cancer Epidemiol Biomarkers Prev. 18:2068–73. DOI: 10.1158/1055-9965.EPI-09-0353. PMID: 19531676.

Fig. 1
Peripheral blood and bone marrow findings of primary bone marrow B-cell non-Hodgkin lymphoma with essential thrombocythemia. (A) Atypical lymphocytes with abundant cytoplasm and dense chromatin, and thrombocytosis on the peripheral blood smear (Wright-Giemsa stain, ×100). (B) Atypical lymphocyte with a cleaved nucleus on the peripheral blood smear (Wright-Giemsa stain, ×100). (C) Medium-sized atypical lymphocytes with an occasional indented or a cleaved nucleus on the bone marrow aspiration smear (Wright-Giemsa stain, ×100). (D) Atypical lymphocyte with a cleaved nucleus on the bone marrow aspiration smear (Wright-Giemsa stain, ×100). (E) Markedly increased number of mature megakaryocytes with hyperlobulated nuclei often forming clusters (hematoxylin and eosin, ×20). (F) Paratrabecular infiltrate of B-cell non-Hodgkin lymphoma in the bone marrow biopsy section (BCL2 stain, ×20).
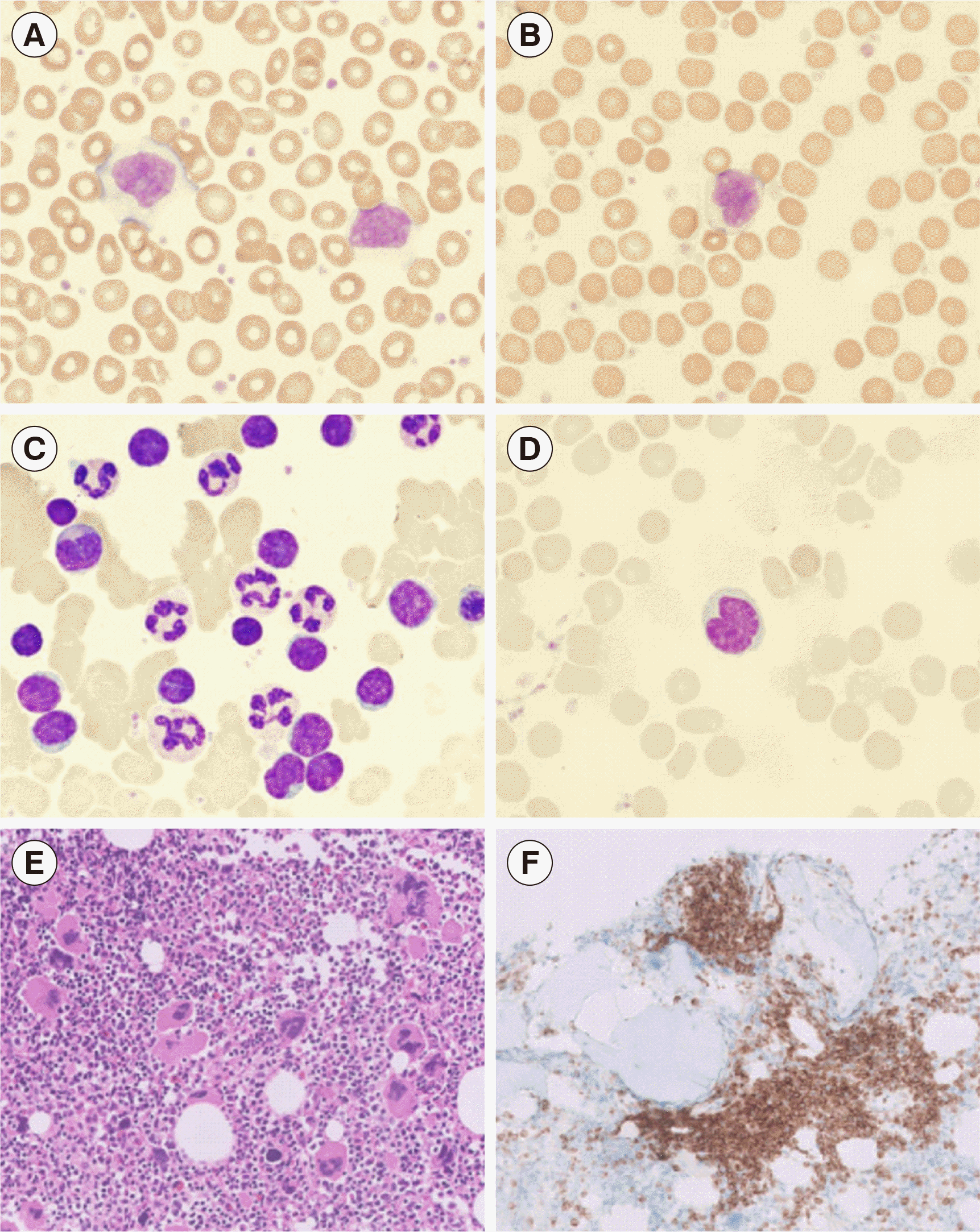
Fig. 2
Identification of somatic variants from bone marrow aspirates using the Integrative Genomics Viewer (Broad Institute, Cambridge, MA, USA). A custom targeted panel sequenced on the NextSeq 550Dx platform (Illumina, San Diego, CA, USA) detected (A) MYD88 p.Val217Phe (NM_002468.4:c.649G>T) at a variant allele frequency (VAF) of 17.5% and (B) JAK2 p.Val617Phe (NM_004972.3:c.1849G>T) at a VAF of 16.3%. (C) Trisomy 12 identified from off-target read analysis using next-generation sequencing (arrow). Data normalization for copy number variation was performed using germline data from skin fibroblast-derived DNA.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download