INTRODUCTION
MATERIALS AND METHODS
Instruments and test items
Sample preparation for comparability assessment
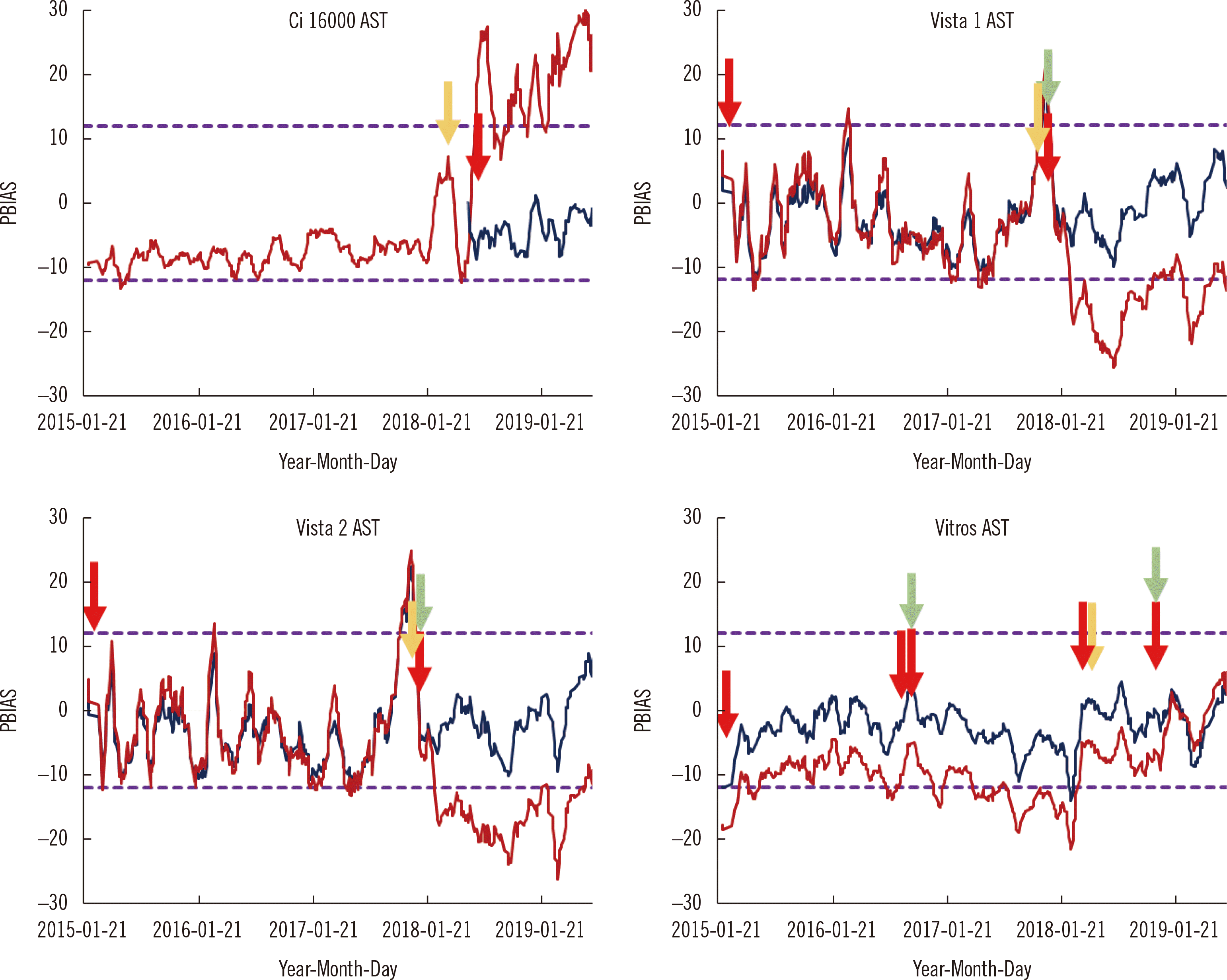
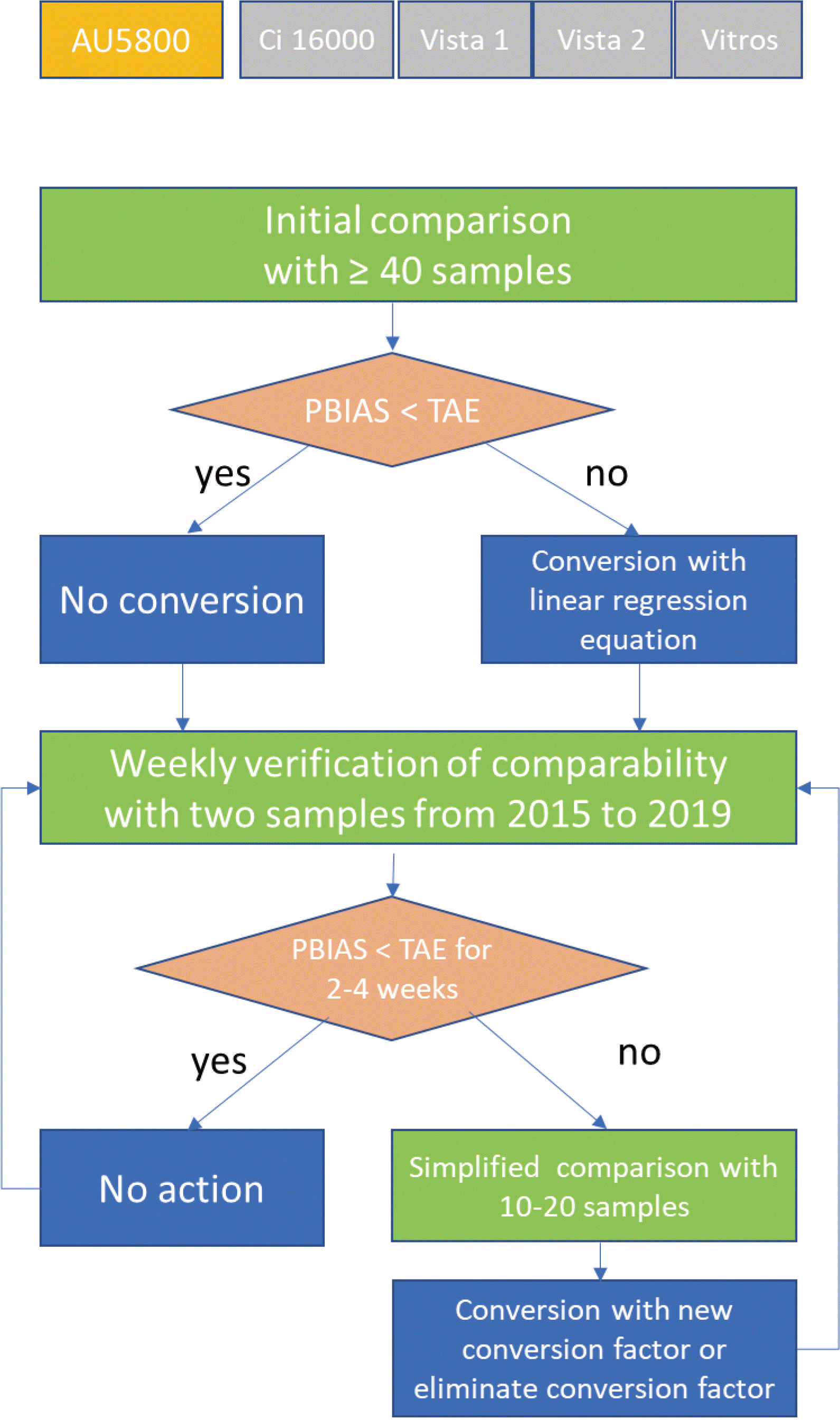
Comparability assessment protocol
Initial comparison and conversion action
Weekly comparability verification
Simplified comparison
Categorization of the results and statistical analysis
Weekly comparability verification and simplified comparison
Table 1
*These parameters were used as the acceptance criteria and allowable limits for the within-laboratory comparability verification of multiple instruments. The allowable limits have fixed deviations (+/−) from the target or consensus values up to a particular value (To) and proportional deviations (TAE) at higher values.
Abbreviations: AST, aspartate transaminase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; ALB, albumin; TP, total protein; Na, sodium; K, potassium; Cl, chloride; Ca, calcium; P, phosphate; Chol, cholesterol; Cr, creatinine; TAE, total allowable error; RCPAQAP, Royal College of Pathologists of Australasia Quality Assurance Program.
RESULTS
Initial comparison
Trendlines of weekly comparability verification
Fig. 2
Distribution of absolute PBIAS and PNR
Table 2
| Analyte | Instrument | Measured data without conversion action* | Data before (measured)† and after (converted) conversion action | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%)‡ | Average absolute PBIAS (95% CI)§ |
PNR|| (% PNR) |
N (%) | Measured results | Converted results | ||||
| Average absolute PBIAS (95% CI)§ |
PNR (% PNR) |
Average absolute PBIAS (95% CI) |
PNR (% PNR) |
||||||
| AST | Ci16000¶ | 338 (78) | 8.1 (7.5–8.6) | 1.5 (1.2) | 94 (22) | 49.6 (48.3–51.0) | 64.9 (14.1) | 7.1 (5.6–8.6) | 3.2 (0.7) |
| Vista1¶ | 0 (0) | 432 (100) | 11.6 (10.4–12.8) | 25.5 (25.5) | 7.2 (6.4–8.1) | 3.9 (3.9) | |||
| Vista2¶ | 0 (0) | 432 (100) | 12.1 (10.8–13.3) | 26.6 (26.6) | 7.6 (6.7–8.5) | 5.1 (5.1) | |||
| Vitros¶ | 2 (0) | 12.5 | 0.0 (0.0) | 430 (100) | 9.9 (9.3–10.5) | 19.5 (19.4) | 4.9 (4.3–5.4) | 0.9 (0.9) | |
| ALT | Ci16000¶ | 401 (93) | 5.2 (4.7–5.8) | 0.0 (0.0) | 31 (7) | 104.3 | 67.7 (4.9) | 23.4 | 29.0 (2.1) |
| Vista1¶ | 0 (0) | 432 (100) | 31.6 (29.9–33.4) | 75.5 (75.5) | 9.7 (8.5–10.8) | 2.3 (2.3) | |||
| Vista2¶ | 0 (0) | 432 (100) | 30.8 (29.2–32.4) | 72.5 (72.5) | 9.3 (8.2–10.4) | 2.5 (2.5) | |||
| Vitros¶ | 2 (0) | 9.3 | 0.0 (0.0) | 430 (100) | 14.7 (13.4–16.0) | 23.7 (23.6) | 9.0 (7.9–10.1) | 1.4 (1.4) | |
| ALP | Ci16000¶ | 98 (23) | 5.1 (4.4–5.7) | 0.0 (0.0) | 334 (77) | 12.5 (11.6–13.4) | 22.8 (17.6) | 4.0 (3.5–4.5) | 0.0 (0.0) |
| Vista1¶ | 0 (0) | 432 (100) | 4.5 (4.1–4.9) | 1.2 (1.2) | 4.1 (3.6–4.5) | 0.0 (0.0) | |||
| Vista2¶ | 0 (0) | 432 (100) | 4.6 (4.3–5.0) | 1.9 (1.9) | 4.2 (3.7–4.7) | 0.7 (0.7) | |||
| Vitros¶ | 0 (0) | 432 (100) | 9.2 (8.5–9.9) | 24.1 (24.1) | 7.2 (6.5–7.9) | 5.1 (5.1) | |||
| ALB | Ci16000¶ | 372 (86) | 2.3 (2.0–2.6) | 3.5 (3.0) | 60 (14) | 16.5 (16.2–16.7) | 11.7 (1.6) | 4.7 (3.9–5.5) | 23.3 (3.2) |
| Vista1¶ | 18 (4) | 4.6 | 27.8 (1.2) | 414 (96) | 7.3 (6.9–7.6) | 58.9 (56.5) | 3.5 (3.2–3.8) | 12.8 (12.3) | |
| Vista2¶ | 18 (4) | 5.7 | 44.4 (1.9) | 414 (96) | 7.6 (7.2–7.9) | 60.4 (57.9) | 3.3 (3.0–3.6) | 11.4 (10.9) | |
| Vitros¶ | 169 (39) | 3.0 (2.4–3.5) | 6.5 (2.5) | 263 (61) | 5.3 (4.9–5.7) | 17.1 (10.4) | 3.3 (2.9–3.7) | 8.0 (4.9) | |
| TP | Ci16000¶ | 372 (86) | 1.4 (1.2–1.5) | 0.0 (0.0) | 60 (14) | 9.7 (9.5–9.8) | 0.0 (0.0) | 1.4 (1.0–1.8) | 0.0 (0.0) |
| Vista1¶ | 292 (68) | 1.8 (1.6–2.1) | 2.7 (1.9) | 140 (32) | 6.3 (6.1–6.5) | 12.9 (4.2) | 3.0 (2.6–3.3) | 12.9 (4.2) | |
| Vista2¶ | 292 (68) | 1.5 (1.2–1.7) | 1.4 (0.9) | 140 (32) | 5.3 (5.1–5.5) | 7.9 (2.5) | 3.1 (2.7–3.4) | 8.6 (2.8) | |
| Vitros¶ | 290 (67) | 1.6 (1.4–1.8) | 2.4 (1.6) | 142 (33) | 5.4 (5.2–5.6) | 7.7 (2.5) | 2.5 (2.1–2.9) | 4.9 (1.6) | |
| Na | Ci16000 | 432 (100) | 1.0 (0.9–1.1) | 0.9 (0.9) | 0 (0) | ||||
| Vista1¶ | 294 (68) | 1.0 (0.9–1.1) | 3.4 (2.3) | 138 (32) | 2.9 (2.8–3.0) | 2.9 (0.9) | 1.1 (0.9–1.2) | 3.6 (1.2) | |
| Vista2¶ | 294 (68) | 1.0 (0.9–1.2) | 5.4 (3.7) | 138 (32) | 3.1 (3.0–3.2) | 9.4 (3.0) | 1.2 (1.0–1.4) | 5.1 (1.6) | |
| Vitros¶ | 398 (92) | 1.1 (1.0–1.3) | 6.5 (6.0) | 34 (8) | 15.7 | 55.9 (4.4) | 1.0 | 2.9 (0.2) | |
| K | Ci16000 | 432 (100) | 1.1 (0.9–1.2) | 0.2 (0.2) | 0 (0) | ||||
| Vista1 | 432 (100) | 2.0 (1.9–2.2) | 0.0 (0.0) | 0 (0) | |||||
| Vista2 | 432 (100) | 2.0 (1.9–2.2) | 0.7 (0.7) | 0 (0) | |||||
| Vitros¶ | 0 (0) | 432 (100) | 2.1 (1.9–2.3) | 3.7 (3.7) | 1.4 (1.2–1.6) | 0.5 (0.5) | |||
| Cl | Ci16000 | 432 (100) | 0.9 (0.8–1.0) | 0.7 (0.7) | 0 (0) | ||||
| Vista1¶ | 4 (1) | 3.8 | 75.0 (0.7) | 428 (99) | 2.3 (2.2–2.5) | 28.0 (27.8) | 1.2 (1.0–1.3) | 0.9 (0.9) | |
| Vista2¶ | 4 (1) | 2.4 | 0.0 (0.0) | 428 (99) | 2.1 (1.9–2.2) | 21.0 (20.8) | 1.3 (1.1–1.4) | 2.6 (2.5) | |
| Vitros¶ | 10 (3) | 12.5 | 20.0 (0.6) | 306 (97) | 1.3 (1.1–1.4) | 3.6 (3.5) | 1.2 (1.0–1.4) | 2.6 (2.5) | |
| Ca | Ci16000 | 198 (46) | 1.6 (1.3–1.8) | 2.0 (0.9) | 234 (54) | 3.6 (3.4–3.8) | 10.3 (5.6) | 1.8 (1.5–2.0) | 2.6 (1.4) |
| Vista1¶ | 0 (0) | 432 (100) | 4.1 (3.8–4.4) | 45.8 (45.8) | 1.9 (1.7–2.1) | 5.6 (5.6) | |||
| Vista2¶ | 0 (0) | 432 (100) | 4.3 (4.0–4.6) | 48.4 (48.4) | 2.0 (1.8–2.2) | 6.3 (6.3) | |||
| Vitros¶ | 0 (0) | 432 (100) | 5.7 (5.5–6.0) | 66.9 (66.9) | 2.1 (1.8–2.3) | 8.6 (8.6) | |||
| P | Ci16000 | 432 (100) | 3.9 (3.6–4.2) | 0.0 (0.0) | 0 (0) | ||||
| Vista1¶ | 0 (0) | 432 (100) | 2.9 (2.5–3.2) | 0.0 (0.0) | 4.4 (4.1–4.7) | 0.0 (0.0) | |||
| Vista2¶ | 0 (0) | 432 (100) | 3.0 (2.7–3.4) | 0.0 (0.0) | 4.4 (4.0–4.7) | 0.2 (0.2) | |||
| Vitros¶ | 0 (0) | 432 (100) | 10.0 (9.5–10.5) | 11.3 (11.3) | 4.6 (4.1–5.0) | 0.2 (0.2) | |||
| Chol | Ci16000¶ | 0 (0) | 432 (100) | 2.3 (2.0–2.6) | 0.0 (0.0) | 2.2 (2.0–2.5) | 0.0 (0.0) | ||
| Vista1¶ | 378 (88) | 3.0 (2.7–3.4) | 0.8 (0.7) | 54 (13) | 24.4 (24.0–24.7) | 3.7 (0.5) | 4.1 (3.2–5.1) | 0.0 (0.0) | |
| Vista2¶ | 378 (88) | 2.5 (2.2–2.8) | 0.0 (0.0) | 54 (13) | 20.6 (20.3–20.9) | 1.9 (0.2) | 3.5 (2.7–4.3) | 0.0 (0.0) | |
| Vitros¶ | 374 (87) | 2.4 (2.1–2.7) | 0.0 (0.0) | 58 (13) | 18.8 (18.5–19.1) | 0.0 (0.0) | 3.0 (2.0–3.9) | 0.0 (0.0) | |
| Cr | Ci16000¶ | 0 (0) | 432 (100) | 5.9 (5.3–6.5) | 15.8 (15.8) | 4.2 (3.7–4.6) | 2.5 (2.8) | ||
| Vista1 | 296 (69) | 4.1 (3.6–4.7) | 5.7 (3.9) | 136 (31) | 16.0 (15.4–16.6) | 27.2 (8.6) | 4.9 (3.9–5.9) | 5.1 (1.6) | |
| Vista2 | 296 (69) | 3.9 (3.4–4.4) | 1.7 (1.2) | 136 (31) | 14.8 (14.3–15.4) | 19.1 (6.0) | 4.2 (3.4–5.1) | 3.7 (1.2) | |
| Vitros | 432 (100) | 5.1 (4.6–5.5) | 8.1 (8.1) | 0 (0) | |||||
| Average¶ | 179 (42) | 3.7 | 6.9 (1.4) | 250 (58) | 12.8 | 23.8 (17.5) | 4.3 | 4.6 (2.5) | |
*Results that showed acceptable comparability to the reference instrument without a converting action. †Results with repeated unacceptable comparability to the reference instrument before conversion, but more acceptable comparability after conversion. ‡The number of times the comparability tests were performed over five years with percentage relative to all available tests in parentheses. §95% confidence intervals were calculated only when the number of cases was more than 50. ||The percentage of unacceptable, incomparable data outside the total allowable limits relative to the number of data points. The percentage relative to total tests (N=432) is shown in parentheses, which can be calculated by multiplying by the percentage of each number of times to total available tests. ¶Statistically significant difference between results before and after the conversion action (P<0.0001).
Inter-instrument CV before and after conversion action
Table 3
| Analytes | Measured results without conversion action | Original results before conversion action | Converted results after conversion action | Allowable bias* |
|---|---|---|---|---|
| AST | 4.3 (0.8) | 8.6 (1.7) | 4.9 (1.0) | 5.1 |
| ALT | 3.2 (0.3) | 17.0 (1.5) | 7.4 (0.7) | 11.1 |
| ALP | 3.5 (0.8) | 6.0 (1.4) | 4.0 (0.9) | 4.3 |
| ALB | 2.0 (0.6) | 4.0 (1.3) | 2.4 (0.8) | 3.2 |
| TP | 1.3 (0.7) | 1.5 (0.8) | 1.0 (0.5) | 2.0 |
| Na | 0.8 (1.0) | 1.2 (1.5) | 0.7 (0.9) | 0.8 |
| K | 1.3 (0.7) | 1.3 (0.7) | 0.8 (0.4) | 1.9 |
| Cl | 0.6 (0.4) | 1.3 (0.9) | 0.9 (0.6) | 1.4 |
| Ca | 1.0 (0.5) | 3.6 (1.8) | 1.5 (0.8) | 2.0 |
| P | 2.1 (0.8) | 4.6 (1.6) | 3.0 (1.1) | 2.8 |
| Cr | All converted | 5.8 (1.3) | 4.5 (1.0) | 4.4 |
| Chol | All converted | 2.1 (0.6) | 1.7 (0.5) | 3.4 |
*Allowable limits for bias according to European recommendations adopted from Baadenhuijsen, et al. [16].




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download