Abstract
Bacterial infection represents a turning point in the natural history of cirrhosis, causing the development of acute-on-chronic liver failure. It significantly affects the outcome of patients listed for liver transplantation. We report the case of a 57-year-old man who had been regularly treated for hepatitis B virus, alcoholic liver cirrhosis, and hepatic failure. The patient was hospitalized again due to variceal bleeding and hepatic coma. He visited the emergency room with painful anal swelling, dysuria, icteric sclera, and serious abdominal distension. The painful anal swelling and necrosis progressed; thus, he was diagnosed with Fournier’s gangrene. Enterococcus faecium and Candida albicans were detected in the blood. Gangrene wound debris was studied extensively. Despite appropriate antibiotic treatment, vancomycin-resistant enterococcus and C. albicans were continuously present in the blood. Wide debridement of the wound and T-colostomy were performed. After this, norepinephrine and vasopressin were used to maintain stable vital signs. It was difficult to establish a liver transplant operation. Despite repeated bleeding, bacterial infections improved with additional antibiotics. Finally, selected deceased donor liver transplantation in controlled Fournier’s gangrene was successfully performed. Controlled infections may be allowed in transplantation surgery.
Bacterial infection significantly modifies the natural history of cirrhosis patients listed for liver transplantation (LT). Severe infection in an end-stage liver failure patient can result in multi-organ failure involving further deterioration of liver function. Even if this can move the patient up on the waiting list, the patient should be prioritized only after adequate control of the infection. However, the definition of “controlled infection” is lacking. In the setting of LT, patients should be considered suitable for transplantation after resolution of the infection. Frequent hospitalizations and contact with healthcare facilities, immune dysregulation, spontaneous bacterial peritonitis (SBP), varix bleeding, and bloodstream infections are the most common causes of bacterial infection.
As for patients with severe acute-on-chronic liver failure (ACLF) on the waiting list [1,2], prioritization rules with respect to distributive justice, the ideal timing for LT, and delisting criteria should be discussed. According to recent studies, selected patients with “controlled infection” should be considered for transplant because this condition does not impair the posttransplantation outcome [3,4]. Antibiotic prophylaxis is very important for cirrhotic patients with gastrointestinal bleeding, previous episodes of SBP, or repeated intensive care unit (ICU) admission.
Approval for this study of institutional review board was waived because this study is a case report of a single patient and did not include protected health information, data analysis, or testing of a hypothesis, and was de-identified. Informed consent forms were provided to the patients based on the Declaration of Helsinki, and voluntary consent was obtained from the patient.
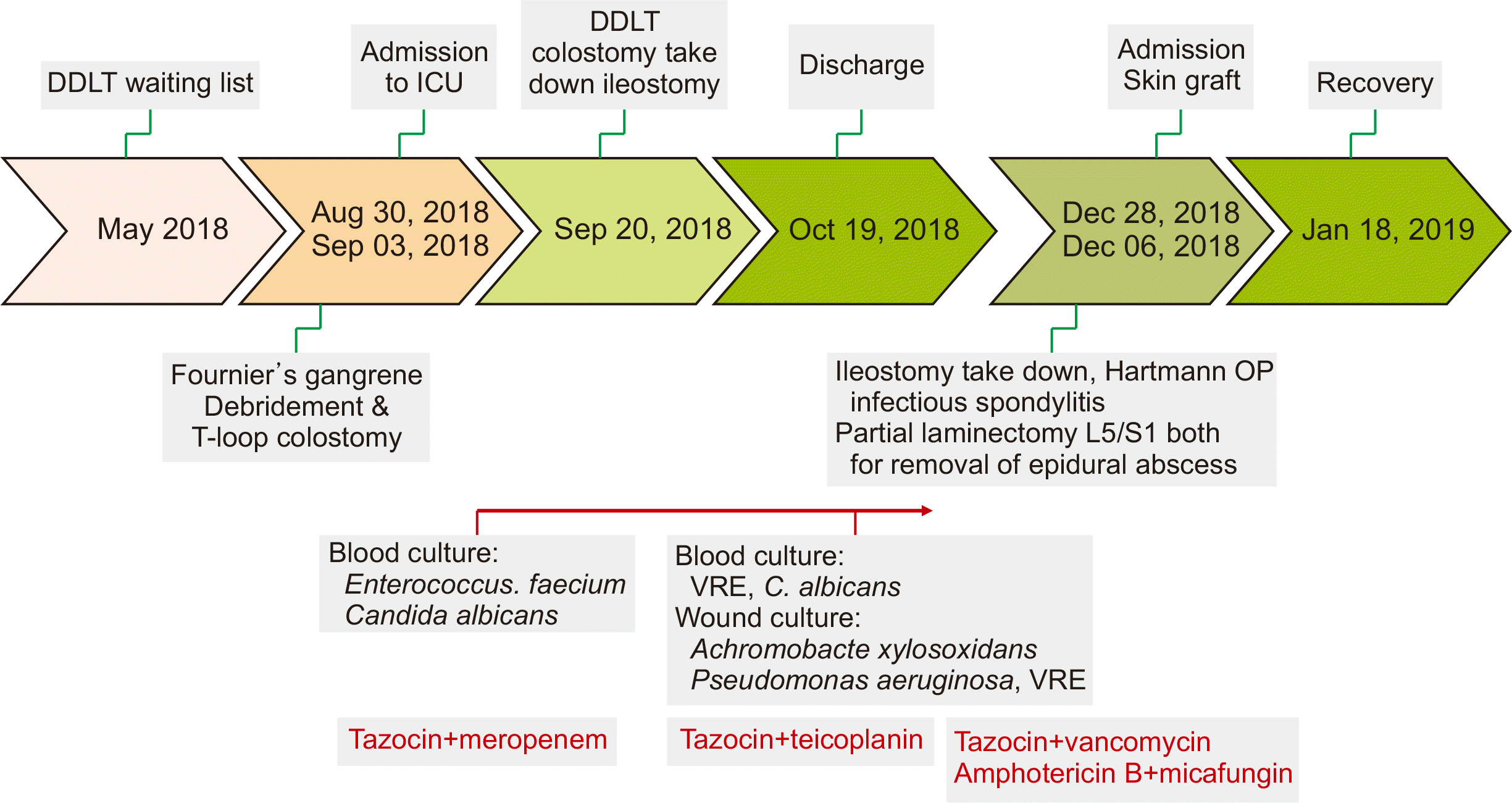
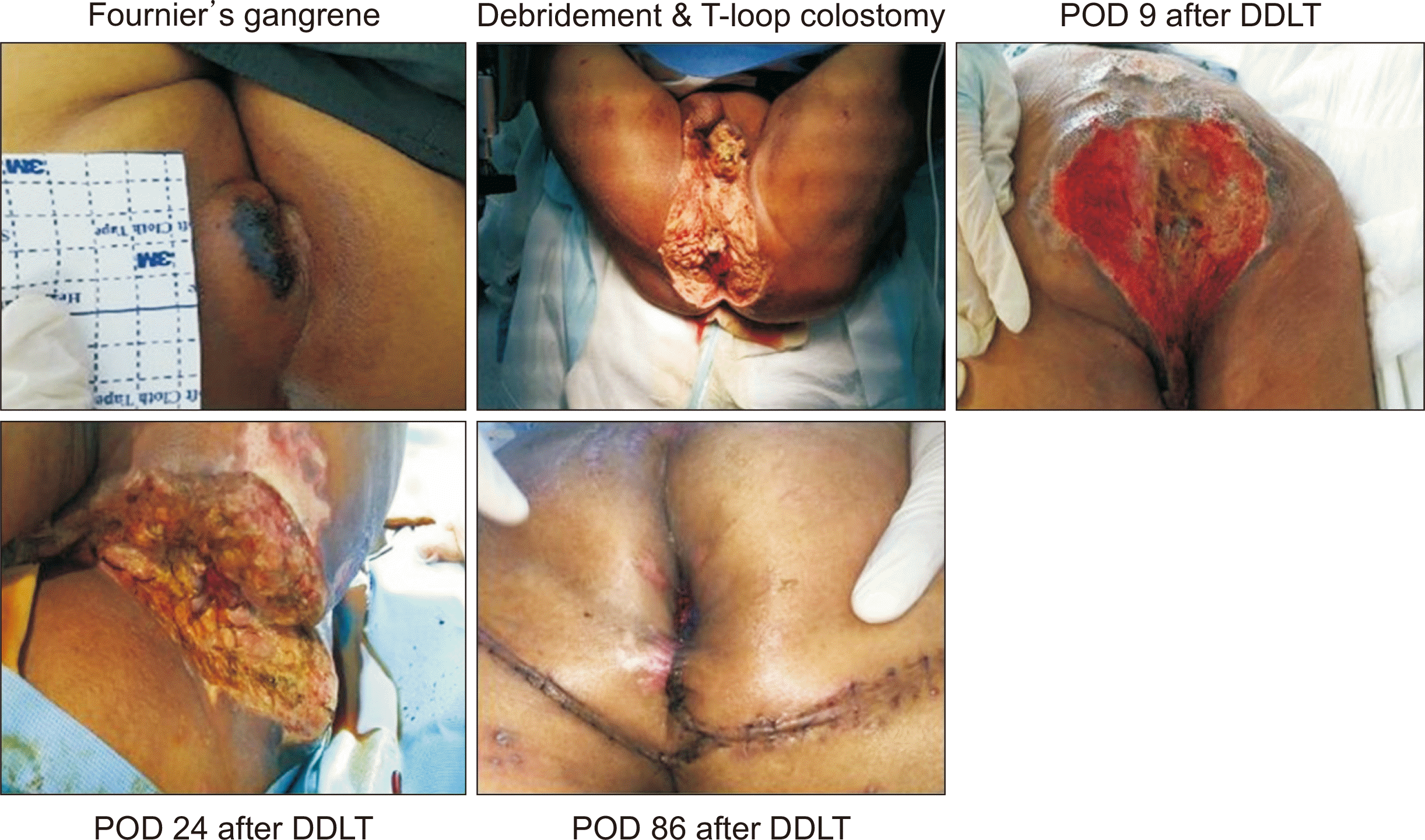
This is the case of a 57-year-old man who had been regularly treated for hepatitis B virus, alcoholic liver cirrhosis, and hepatic failure since March 2015. The patient had esophageal varix bleeding and hepatic encephalopathy (Child-Pugh grade C), which improved with intensive medical care. The patient was hospitalized again due to variceal bleeding and hepatic coma. He also underwent left bipolar hemiarthroplasty because of a left femoral neck fracture in April 2018. The patient’s decompensated liver cirrhosis was aggravated; thus, he was listed as a liver transplant candidate (model for end-stage liver disease [MELD] score of 28 points). In August 2018, he visited the emergency room with painful anal swelling, dysuria, severe icteric sclera, and serious abdominal distension. The hepato-renal syndrome progressed and was admitted to the ICU. The painful anal swelling and necrosis progressed, and he was diagnosed with Fournier’s gangrene. Wide debridement of the wound and T-colostomy were performed (Fig. 1). Since then, bleeding control surgery has been performed. After surgery, norepinephrine and vasopressin were continuously administered to maintain stable vital signs. Bleeding control was repeated. Finally, deceased donor liver transplantation (DDLT) was performed in September 2018 (MELD score of 40 points).
Enterococcus faecium and Candida albicans were detected in the blood. Gangrene wound debris was studied extensively. Despite appropriate antibiotic treatment, vancomycin-resistant Enterococcus (VRE) and C. albicans were continuously present in the blood while Achromobacter xylosoxidans, Pseudomonas aeruginosa, and VRE were present in the wound. Subsequently, varix and wound bleeding due to coagulopathy continued. Despite constant blood transfusions, his hemoglobin level dropped to 3 g/dL. The patient's condition was severe and it was difficult to establish the liver transplant operation. Despite repeated bleeding, bacterial infections had improved with additional antibiotics. This demonstrated that necrotic wound care and appropriate antibiotic treatment can significantly control the infection status. As shown in Fig. 1, tazocin and meropenem were administered at the initial stage of Fournier’s gangrene before transplantation. There was no negative conversion of the bacteria prior to transplantation; therefore, vancomycin was added immediately after the transplantation. In addition to this, tazocin and teicoplanin were administered after the surgery. Amphotericin B and micafungin were used as antifungal agents after transplantation (Fig. 1). C-reactive protein levels also decreased. After DDLT, the patient recovered (Fig. 2). Caution was taken with immunosuppressant treatment. A triple regimen was used, and the FK-506 trough level was adjusted between 4–6 ng/mL to balance infection and immunosuppression. Mycomofetil and steroids were administered at the usual doses.
This is a case in which successful LT was performed in the midst of overcoming septicemia and improving infection. LT for the most severely ill patients with cirrhosis and multiple organ dysfunction remains controversial. ACLF is characterized by acute deterioration of liver function in patients with cirrhosis, in combination with recently defined organ failure.
The liver plays a central role during sepsis and is essential for the regulation of immune defense during systemic infections through mechanisms such as bacterial clearance, acute-phase protein or cytokine production, and metabolic adaptation to inflammation. Hepatic dysfunction substantially impairs the prognosis of sepsis and serves as a powerful independent predictor of mortality in the ICU [5]. Sepsis is particularly problematic in patients with liver cirrhosis who experience variceal bleeding or episodes of SBP, as it can trigger ACLF. Notably, the survival of these patients at 6-month posttransplantation was significantly better than that of patients who were delisted or did not undergo transplantation after infection [6].
Mücke et al. [7] reported that the outcome of patients with bacterial infection-triggered ACLF was distinct from that of non-infection-triggered ACLF (30-day survival, 71.6% vs. 33.8%; P<0.001); furthermore, it was observed that infection-triggered ACLF was independently associated with increased mortality (odds ratio, 4.28; P<0.001). This may indicate the need for immediate treatment of infections in patients with ACLF upon hospitalization [7].
Cirrhotic patients have immunocompromised states and increased susceptibility to developing community and hospital-acquired infections, especially in those awaiting LT. The risk of dropout from the waiting list and the possibility of undergoing LT after recovery from an infection or during a controlled infection should be evaluated. Sepsis-related organ damage in cirrhosis is characterized by an excessive inflammatory response and a decrease in hepatic tolerance [8]. SBP and urinary tract infections are the most frequent bacterial infections in cirrhosis, followed by pneumonia, skin and soft tissue infections, and bloodstream infections [8].
SBP is mainly due to bacterial translocation, especially Gram-negative bacteria (GNB); however, epidemiology is rapidly changing. A multicenter study from Portugal [9], which evaluated patients with severe liver dysfunction (median Child-Pugh class C-10; MELD score 19), recently showed an increase in gram-positive bacteria (GPB, 42%) at the time of SBP diagnosis, with one out of three SBP episodes occurring during hospitalization because of the high number of invasive procedures and quinolone prophylaxis. GNB were more prevalent in community-acquired and healthcare-acquired infections, while GPB was more common in hospital-acquired infections. Enterobacteriaceae (44.3%; particularly Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis) and Enterococci (E. faecium and Enterococcus faecalis) (19.7%) were the pathogens.
Similarly, our patient also had GNB and fungi; specifically, E. faecium, and C. albicans were detected in blood. Despite appropriate antibiotic treatment, VRE and C. albicans were continuously present in the blood while A. xylosoxidans, P. aeruginosa, and VRE were present in the wound. Bacteremia after LT frequently occurs and is a potentially severe complication that affects patient and graft survival. The occurrence of bacteremia after pediatric LT is associated with an increased number of vascular complications and reoperations. Although challenging, proper control of bacterial infections and early LT before developing uncontrolled cholangitis may be useful in reducing vascular complications and unexpected reoperations in patients with biliary atresia [10]. Respiratory multidrug-resistant (MDR) bacteremia is a relative contraindication for lung transplantation. The Kaplan-Meier survival curve showed that patients with early posttransplantation pneumonia had significantly higher 1-year mortality than those without [11].
Frequent varix bleeding and the resulting low blood pressure increase the prevalence of infections in patients with cirrhosis. Variceal bleeding is another predictor for the onset of bacterial infections. According to blood transfusion requirements, mean arterial pressure is an independent predictor of bacterial infection onset [12].
The prognosis of bacterial infection is significantly associated with the severity of liver disease and extrahepatic organ involvement [13,14]. A systematic review [15], 178 different studies with 11,987 patients who have had an episode of bacterial infection reported 1-, 3-, and 12-month mortality rates of 30.3%, 44%, and 63%, respectively. Moreover, almost half of the patients who survived after 1 month died within a year. Several studies have recently investigated the outcome of patients who underwent LT with “controlled” infection. In an Italian study [3], 84 patients were considered eligible for LT after the disappearance of signs and symptoms suggestive of severe sepsis or septic shock. However, patients with previous infections had higher rates of infection (40% vs. 36%, P=0.003) and posttransplant MDR strains (26% vs. 13%, P=0.005).
Bacterial infection is a common cause of impaired liver function in patients with cirrhosis, especially liver transplant candidates. Colonization of MDR bacteria represents another important issue for patients on the waiting list, because of the risk of spreading bacterial infection in the postoperative course and/or after the introduction of immunosuppression.
Accordingly, strategies focusing on the prevention of infections and extrahepatic organ failure are required for patients with cirrhosis on the waiting list. Furthermore, appropriate antibiotics and the standard of care in cirrhotic patients with gastrointestinal bleeding or previous episodes of SBP are very important.
Selected deceased donor LT in controlled Fournier’s gangrene was performed successfully. Controlled infections may be allowed in transplantation surgery because this condition does not impair post-transplant outcomes. Furthermore, transplant candidates may be considered suitable for LT after the resolution of infection.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: NL, MY. Data curation: BGN, BCL. Formal analysis: MY. Methodology: NL, MY. Project administration: BGN, MY. Visualization: BGN, NL. Writing–original draft: NL, MY. Writing–review & editing: MY.
REFERENCES
1. Gustot T, Agarwal B. 2017; Selected patients with acute-on-chronic liver failure grade 3 are not too sick to be considered for liver transplantation. J Hepatol. 67:667–8. DOI: 10.1016/j.jhep.2017.07.017. PMID: 28923205.

2. Putignano A, Gustot T. 2017; New concepts in acute-on-chronic liver failure: implications for liver transplantation. Liver Transpl. 23:234–43. DOI: 10.1002/lt.24654. PMID: 27750389.

3. Bertuzzo VR, Giannella M, Cucchetti A, Pinna AD, Grossi A, Ravaioli M, et al. 2017; Impact of preoperative infection on outcome after liver transplantation. Br J Surg. 104:e172–81. DOI: 10.1002/bjs.10449. PMID: 28121031.

4. Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J, et al. 2017; Liver transplantation in the most severely ill cirrhotic patients: a multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 67:708–15. DOI: 10.1016/j.jhep.2017.06.009. PMID: 28645736.

5. Strnad P, Tacke F, Koch A, Trautwein C. 2017; Liver: guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 14:55–66. DOI: 10.1038/nrgastro.2016.168. PMID: 27924081.
6. Reddy KR, O'Leary JG, Kamath PS, Fallon MB, Biggins SW, Wong F, et al. 2015; High risk of delisting or death in liver transplant candidates following infections: results from the North American Consortium for the Study of End-Stage Liver Disease. Liver Transpl. 21:881–8. DOI: 10.1002/lt.24139. PMID: 25845966.

7. Mücke MM, Rumyantseva T, Mücke VT, Schwarzkopf K, Joshi S, Kempf VA, et al. 2018; Bacterial infection-triggered acute-on-chronic liver failure is associated with increased mortality. Liver Int. 38:645–53. DOI: 10.1111/liv.13568. PMID: 28853199.

8. Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, et al. 2014; Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 60:1310–24. DOI: 10.1016/j.jhep.2014.01.024. PMID: 24530646.

9. Oliveira AM, Branco JC, Barosa R, Rodrigues JA, Ramos L, Martins A, et al. 2016; Clinical and microbiological characteristics associated with mortality in spontaneous bacterial peritonitis: a multicenter cohort study. Eur J Gastroenterol Hepatol. 28:1216–22. DOI: 10.1097/MEG.0000000000000700. PMID: 27391170.
10. Ihn K, Kang JM, Kim EJ, Lee J, Lee JG, Joo DJ, et al. 2020; Postoperative bacteremia is associated with early vascular complications in pediatric liver transplant recipients with biliary atresia. Korean J Transplant. 34(Suppl 1):S123. DOI: 10.4285/ATW2020.OR-1170.

11. Kim T, Yeo HJ, Kim DH, Jang JH, Son E, Jang JO, et al. 2020; Prognostic impact of perioperative sputum colonization on early outcome after lung transplant. Korean J Transplant. 34(Suppl 1):S174. DOI: 10.4285/ATW2020.OP-1155.

12. Fernández J, Ruiz del Arbol L, Gómez C, Durandez R, Serradilla R, Guarner C, et al. 2006; Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 131:1049–56. DOI: 10.1053/j.gastro.2006.07.010. PMID: 17030175.

13. Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. 2013; Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 144:1426–37. DOI: 10.1053/j.gastro.2013.02.042. PMID: 23474284.

14. Bruns T, Zimmermann HW, Stallmach A. 2014; Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 20:2542–54. DOI: 10.3748/wjg.v20.i10.2542. PMID: 24627590. PMCID: PMC3949263.

15. Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. 2010; Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 139:1246–56. DOI: 10.1053/j.gastro.2010.06.019. PMID: 20558165.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download