INTRODUCTION
METHODS
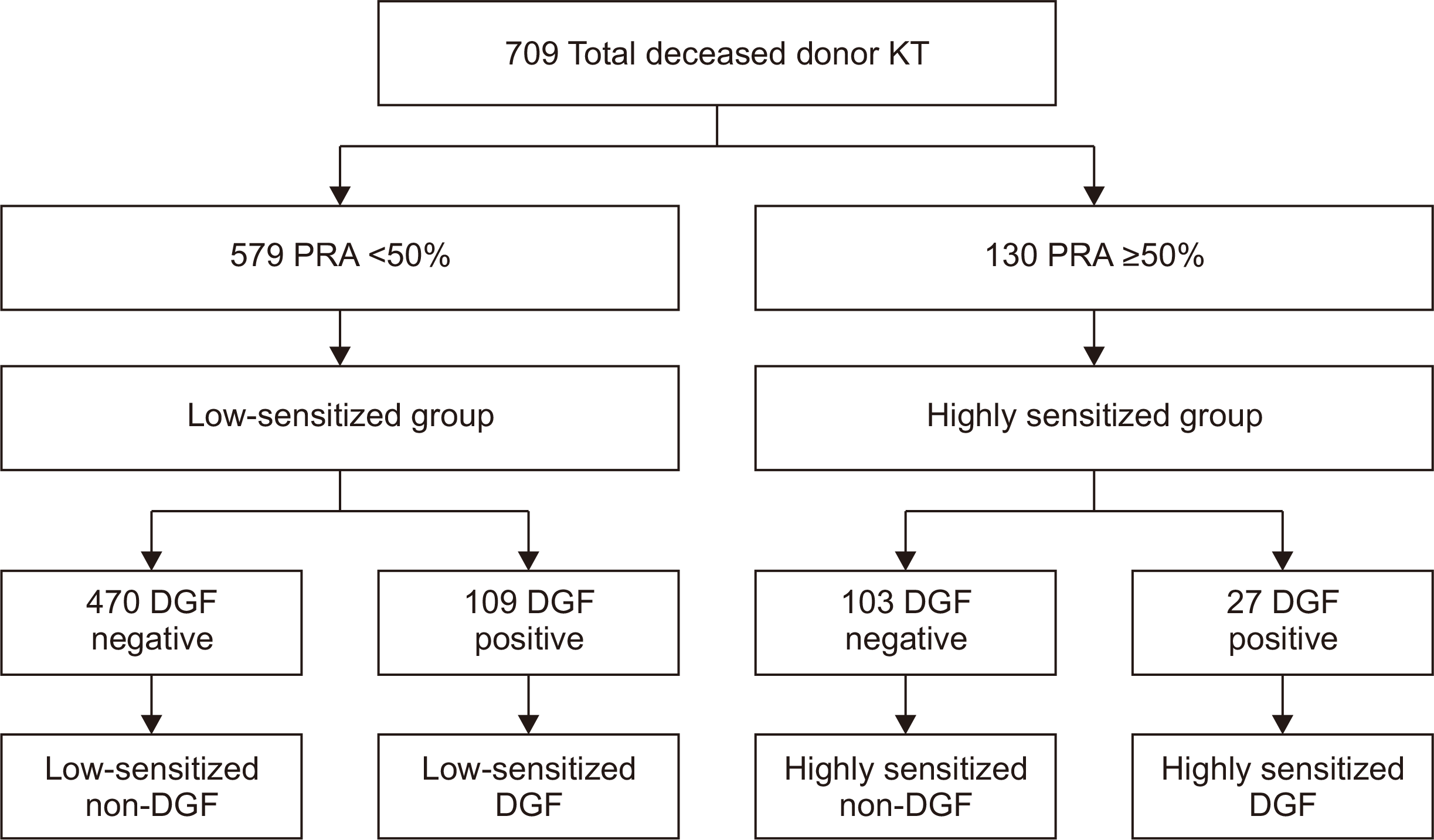
Study Population
Fig. 1
Clinical Parameters and Outcomes
Statistical Analysis
RESULTS
Comparison of Clinical and Laboratory Parameters According to Pre-sensitization and Development of DGF
Table 1
| Variable |
Low-sensitized non-DGF (n=470) |
Low-sensitized DGF (n=109) |
Highly sensitized non-DGF (n=103) |
Highly sensitized DGF (n=27) |
|---|---|---|---|---|
| Recipient | ||||
| Age (yr) | 49.8±9.8 | 50.5±10.0 | 50.1±9.8 | 52.6±9.1 |
| Elderly (≥65 yr) | 88 (18.7) | 15 (13.8) | 22 (21.4) | 6 (22.2) |
| Male sex | 297 (63.2)c) | 65 (59.6)c) | 45 (43.7)a),b) | 12 (44.4) |
| BMI (kg/m2) | 23.1±4.8 | 22.6±4.6 | 23.0±3.5 | 23.0±3.1 |
| Dialysis vintagee) | 8.1±9.5 | 9.2±11.6 | 10.0±12.2 | 36.8±34.9 |
| Primary renal disease | ||||
| DM | 90 (19.1) | 18 (16.5) | 12 (11.7) | 4 (14.8) |
| HTN | 88 (18.7) | 18 (16.5) | 13 (12.6) | 2 (7.4) |
| CGN | 198 (42.1) | 52 (47.7) | 61 (59.2) | 19 (70.4) |
| Others | 94 (20.0) | 21 (19.3) | 17 (16.5) | 2 (7.4) |
| History of HTN | 374 (79.6)d) | 90 (82.6)d) | 79 (76.7) | 17 (63.0)a),b) |
| History of DM | 101 (21.5) | 25 (22.9) | 14 (13.6) | 7 (25.9) |
| Previous KT | 28 (6.0)c),d) | 10 (9.2)c),d) | 33 (32.0)a),b) | 12 (44.4)a),b) |
| Donor | ||||
| Age (yr) | 46.0±14.4 | 45.1±14.3 | 45.9±14.3 | 45.9±13.0 |
| Elderly (age≥ 65 yr) | 88 (18.7)b) | 10 (9.2)a) | 13 (12.6) | 3 (11.1) |
| Male sex | 309 (65.7)c) | 75 (68.8) | 78 (75.7)a) | 21 (77.8) |
| BMI (kg/m2) | 23.4±3.8 | 23.6±3.5 | 23.5±3.5 | 23.0±3.0 |
| HTN | 99 (21.1) | 21 (19.3) | 15 (14.6) | 3 (11.1) |
| DM | 49 (10.4) | 7 (6.4) | 7 (6.8) | 3 (11.1) |
| Cold ischemic time (hr) | 4.2±2.1 | 4.8±2.2c) | 3.9±2.1b) | 4.5±2.4 |
| SCD vs. ECDf) | ||||
| SCD | 318 (67.7) | 80 (73.4) | 78 (75.7) | 21 (77.8) |
| ECD | 152 (32.3) | 29 (26.6) | 25 (24.3) | 6 (22.2) |
| AKI by KDIGO | 240 (51.1)b),d) | 76 (69.7)a),c) | 48 (46.6)b),d) | 20 (74.1)a),c) |
| KDPI (%) | 62.8±25.9 | 62.9±22.2 | 59.0±24.5 | 63.5±23.1 |
| Immunologic parameter | ||||
| Mismatch number | ||||
| <3 | 99 (21.1) | 17 (15.6)d) | 18 (17.5) | 9 (33.3)b) |
| ≥3 | 371 (78.9) | 92 (84.4)d) | 85 (82.5) | 18 (66.7)b) |
| HLA-DSA | 7/434 (1.6)c),d) | 4/88 (4.5)c),d) | 24/100 (24.0)a),b) | 5/24 (20.8)a),b) |
| class I | 2/434 (0.5)c),d) | 0/88 (0.0)c),d) | 9/100 (9.0)a),b) | 3/24 (12.5)a),b) |
| class II | 5/434 (1.2)b),c),d) | 4/88 (4.5)a),c) | 18/100 (18.0)a),b) | 4/24 (16.7)a) |
| HLA-DSA, MFI | ||||
| 1,000 to <3,000 | 2/434 (0.5) | 4/88 (4.5) | 5/100 (5.0) | 2/24 (8.3) |
| 3,000 to <5,000 | 1/434 (0.2) | 0/88 (0.0) | 10/100 (10.0) | 0/24 (0.0) |
| 5,000 to <10,000 | 3/434 (0.7) | 0/88 (0.0) | 4/100 (4.0) | 1/24 (4.2) |
| ≥10,000 | 1/434 (0.2) | 0/88 (0.0) | 5/100 (5.0) | 2/24 (8.3) |
| Mean MFI | 3244.5c),d) | 2401.7c),d) | 7199.7a),b) | 7391.4a),b) |
| Induction therapy | ||||
| Basiliximab | 336 (71.5)b),c),d) | 67 (61.5)a),c) | 35 (34.0)a),b) | 12 (44.4)a) |
| ATG | 134 (28.5)b),c),d) | 42 (38.5)a),c) | 68 (66.0)a),b) | 15 (55.6)a) |
| Combined rituximab | 2 (0.4)c),d) | 1 (0.9)c),d) | 41 (39.8)a),b) | 7 (25.9)a),b) |
| Maintenance immunosuppression | ||||
| Tac-MMF/Myf-steroid | 468 (99.6) | 107 (98.2) | 103 (100.0) | 27 (100.0) |
| CSA-MMF/Myf-steroid | 2 (0.4) | 1 (0.9) | 0 | 0 |
| SRL-MMF/Myf-steroid | 0 | 1 (0.9) | 0 | 0 |
Values are presented as mean±standard deviation or number (%). The values for HLA-DSA were only for patients who had undergone HLA-DSA testing. In addition, the number of patients undergoing HLA-DSA test in each group was expressed in denominator.
DGF, delayed graft function; BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; CGN, chronic glomerulonephritis; KT, kidney transplantation; SCD, standard criteria donors; ECD, expanded criteria donors; AKI, acute kidney injury; KDIGO, kidney disease improving global outcomes; KDPI, kidney donor profile index; HLA, human leukocyte antigen; DSA, donor-specific antibody; MFI, mean fluorescence intensity; ATG, antithymocyte globulin; Tac, tacrolimus; MMF, mycophenolate mofetil; Myf, mycophenolic acid; CSA, cyclosporine A; SRL, sirolimus.
a)P<0.05 vs. low-sensitized non-DGF; b)P<0.05 vs. low-sensitized DGF; c)P<0.05 vs. highly sensitized non-DGF; d)P<0.05 vs. highly sensitized DGF; e)For patients with a previous kidney transplant history, the years from the restart of dialysis to the date of kidney transplant was defined as the dialysis vintage; f)ECD was defined as all donors older than 60 years and donors older than 50 years with any two of the following criteria: (1) hypertension, (2) cerebrovascular cause of brain death, or (3) pre-retrieval serum creatinine level >1.5 mg/dL [20].
Comparison of BPAR According to Pre-sensitization and Development of DGF
Fig. 2
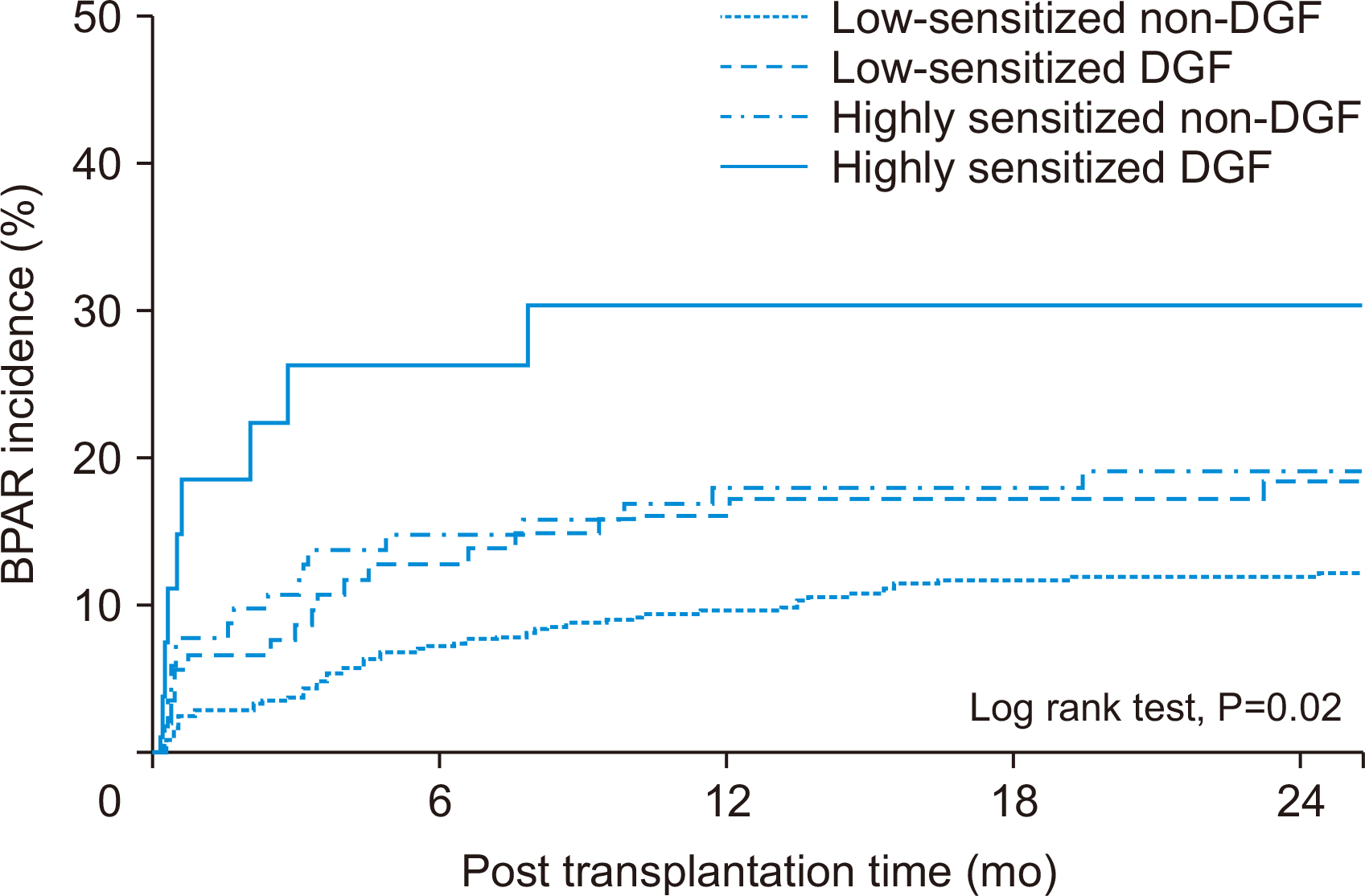
Table 2
| Variable |
Low-sensitized non-DGF |
Low-sensitized DGF |
Highly sensitized non-DGF |
Highly sensitized DGF |
|---|---|---|---|---|
| BPAR | 57 (12.1)b),c) | 20 (18.3) | 21 (20.4)a) | 8 (29.6)a) |
| BPAR type | ||||
| TCMR | 38 (66.7) | 14 (70.0) | 6 (28.6) | 4 (50.0) |
| ABMR | 9 (15.8) | 2 (10.0) | 6 (28.6) | 1 (12.5) |
| Mixed | 6 (10.5) | 1 (5.0) | 0 | 2 (25.0) |
| Others | 4 (7.0) | 3 (15.0) | 9 (42.9) | 1 (12.5) |
| TCMR including mixed rejection | 44 (9.4)c) | 15 (13.8) | 6 (5.8)c) | 6 (22.2)a),b) |
| ABMR including mixed rejection | 15 (3.2) | 3 (2.8) | 6 (5.8) | 3 (11.1) |
Values are presented as number (%); the percentage in the BPAR type is for the total number of BPARs. A total of 303 KTRs underwent allograft biopsy, of which BPAR was identified in 106 KTRs (35.0%). This is the result of BPAR which was confirmed at least once in KTRs who underwent repeated biopsy.
Table 3
| Variable | Crude model | Adjusted modela) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Low-sensitized non-DGF | Reference | Reference | |||||
| Low-sensitized DGF | 1.628 | 0.931–2.846 | 0.087 | 1.532 | 0.871–2.692 | 0.138 | |
| Highly sensitized non-DGF | 1.856 | 1.067–3.228 | 0.029 | 1.866 | 1.065–3.269 | 0.029 | |
| Highly sensitized DGF | 3.051 | 1.277–7.291 | 0.012 | 3.631 | 1.481–8.904 | 0.005 | |
| Elderly recipient | - | - | - | 0.451 | 0.232–0.877 | 0.019 | |
| MN (≥3) | - | - | - | 2.521 | 1.295–4.904 | 0.006 | |
BPAR, biopsy proven acute rejection; HR, hazard ratio; CI, confidence interval; DGF, delayed graft function; MN, mismatch number.
a)Adjusted by several recipient and donor factors. Recipient factors included sex, age, history of diabetes mellitus, previous kidney transplant history, and human leukocyte antigen mismatch number. And donor factors included sex, age, and history of diabetes mellitus. Elderly was defined as 65 years or older.
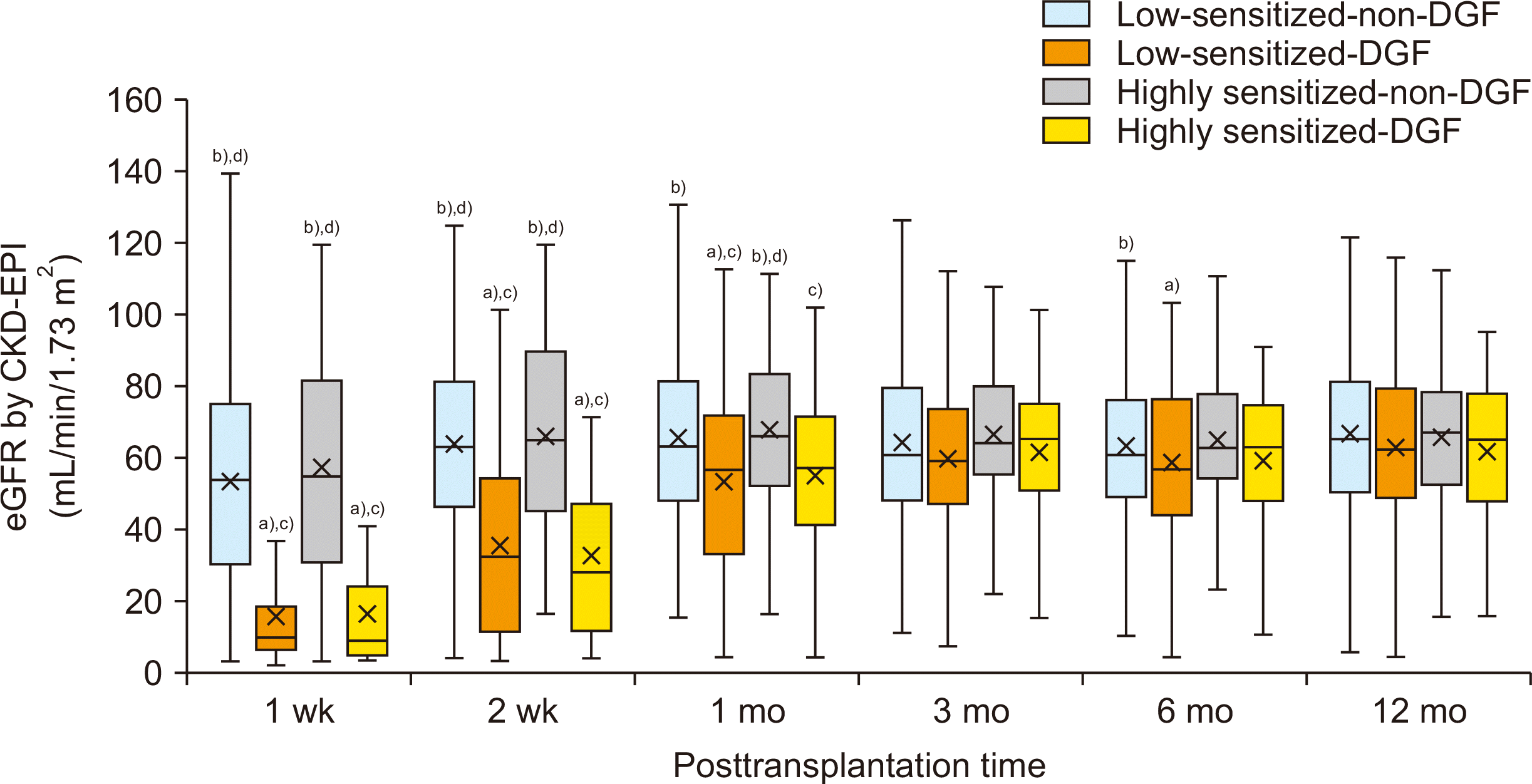
Comparison of Allograft Function According to Pre-sensitization and Development of DGF
Fig. 3
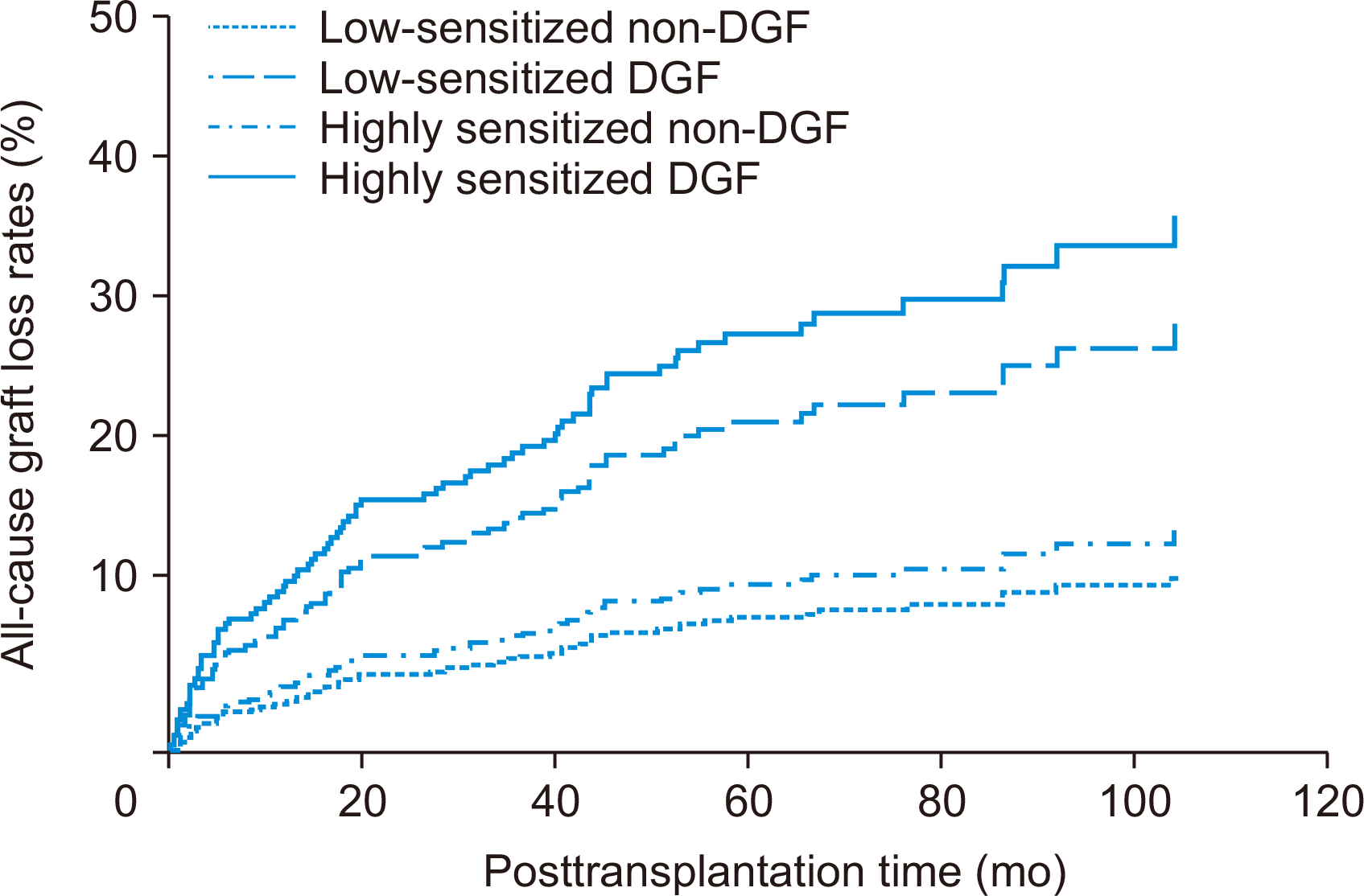
Comparison of Death-Censored Allograft Survival According to Pre-sensitization and Development of DGF
Fig. 4
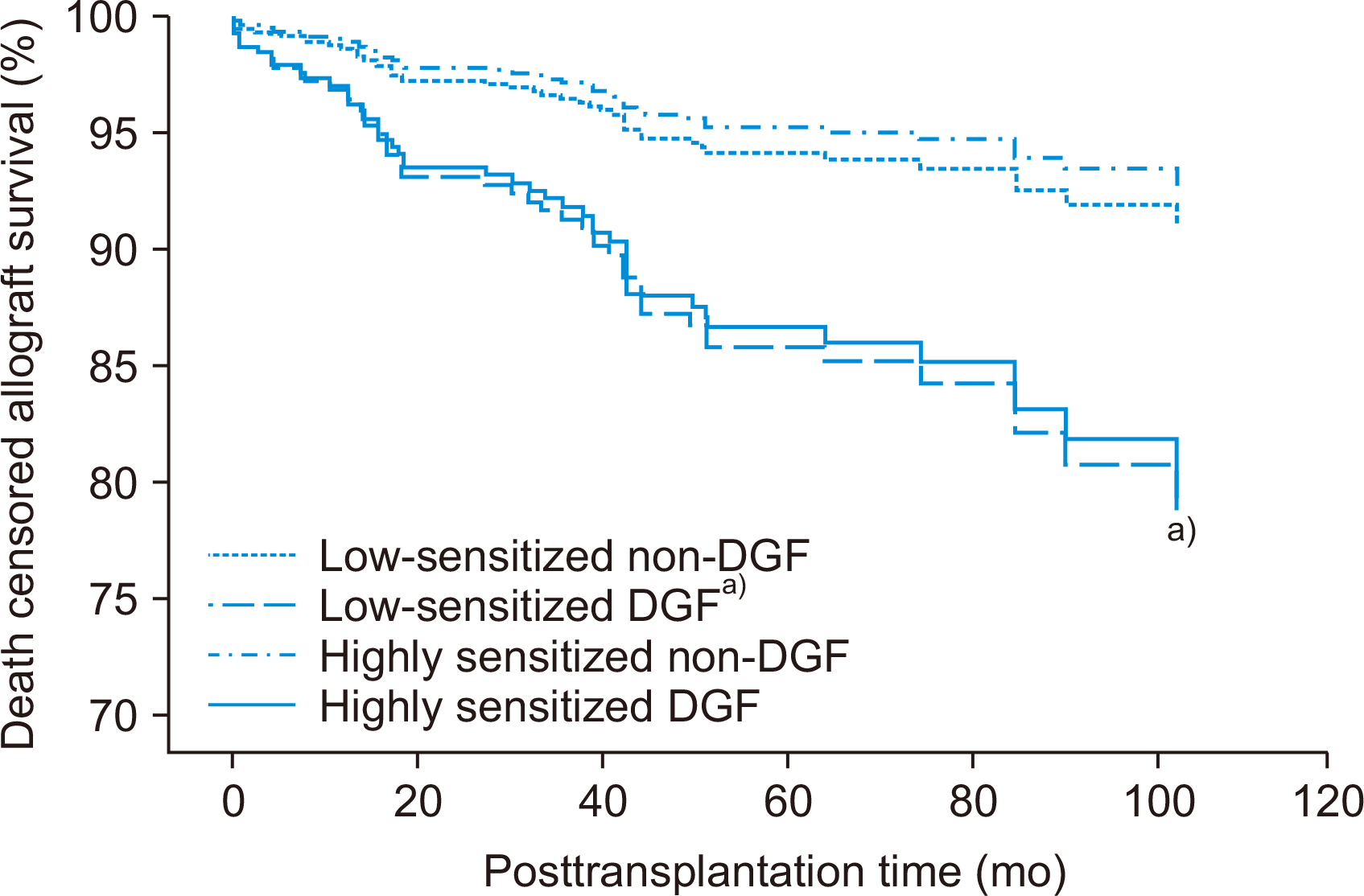
Table 4
| Crude model | Adjusted modela) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| Death-censored allograft survival | - | - | - | - | - | - | |
| Low-sensitized non-DGF | Reference | Reference | |||||
| Low-sensitized DGF | 2.352 | 1.226–4.510 | 0.010 | 2.523 | 1.307–4.872 | 0.006 | |
| Highly sensitized non-DGF | 0.776 | 0.270–2.227 | 0.637 | 0.805 | 0.280–2.312 | 0.686 | |
| Highly sensitized DGF | 2.281 | 0.689–7.545 | 0.177 | 2.366 | 0.714–7.835 | 0.159 | |
| Patient mortality | - | - | - | - | - | - | |
| Low-sensitized non-DGF | - | Reference | - | - | - | - | |
| Low-sensitized DGF | 2.173 | 0.974–4.848 | 0.058 | - | - | - | |
| Highly sensitized non-DGF | 1.363 | 0.505–3.676 | 0.541 | - | - | - | |
| Highly sensitized DGF | 2.977 | 0.877–10.107 | 0.080 | - | - | - | |
Comparison of Patient Mortality According to Pre-sensitization and Development of DGF
Fig. 5




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download