INTRODUCTION
Peritoneal tuberculosis (TB) is not uncommon in India, unlike the West, and is a great clinical masquerader.
1,2 Cirrhosis is a significant risk factor associated with peritoneal TB, and the co-occurrence of both is not unusual in clinical practice.
3-5 Recent reports suggest that more peritoneal TB patients have underlying cirrhosis than older studies.
6-8 The gold standard for a diagnosis of peritoneal TB is a demonstration of acid-fast bacilli (AFB) staining and the growth of
Mycobacterium Tuberculosis on the culture of ascitic fluid (AF) or a peritoneal biopsy sample.
9 Unfortunately, a demonstration of AFB in the ascitic fluid has a sensitivity of only 3%, which can increase only up to 34% by culture. Nevertheless, culture also is a labor-intensive and time-consuming process.
9 The diagnostic tool of choice for peritoneal TB is diagnostic laparoscopy with peritoneal biopsy. The laparoscopy findings themselves are highly suggestive and can be taken as synonymous with tuberculosis. More importantly, it provides tissue that can either show histopathological findings or mycobacterial analysis definitive of tuberculosis.
9-12 On the other hand, laparoscopy is an invasive procedure, not routinely available everywhere, and is also associated with a small risk of morbidity and mortality, which may be even higher in patients with cirrhosis.
13-16 A delay in the diagnosis of peritoneal TB increases morbidity and mortality. Therefore, there is a need for a rapid and accurate test.
6 The adenosine deaminase (ADA) level in the ascitic fluid is a good alternative for peritoneoscopy with negligible risk. In meta-analyses, ADA showed excellent sensitivity (93-100%) and specificity (95-97%) in peritoneal TB.
17-19 Despite this, its utility in patients with cirrhosis for diagnosing peritoneal TB is still debatable. There are only a few reports with a small sample size and have yielded contradictory results, emphasizing further exploration of the utility of ADA in these patients.
20-24
Considering its easy availability, cost-effectiveness, and rapid results, this study evaluated the diagnostic performance of ascitic fluid ADA for peritoneal TB, which was confirmed by peritoneal biopsy, especially in patients with cirrhosis.
SUBJECTS AND METHODS
1. Patients
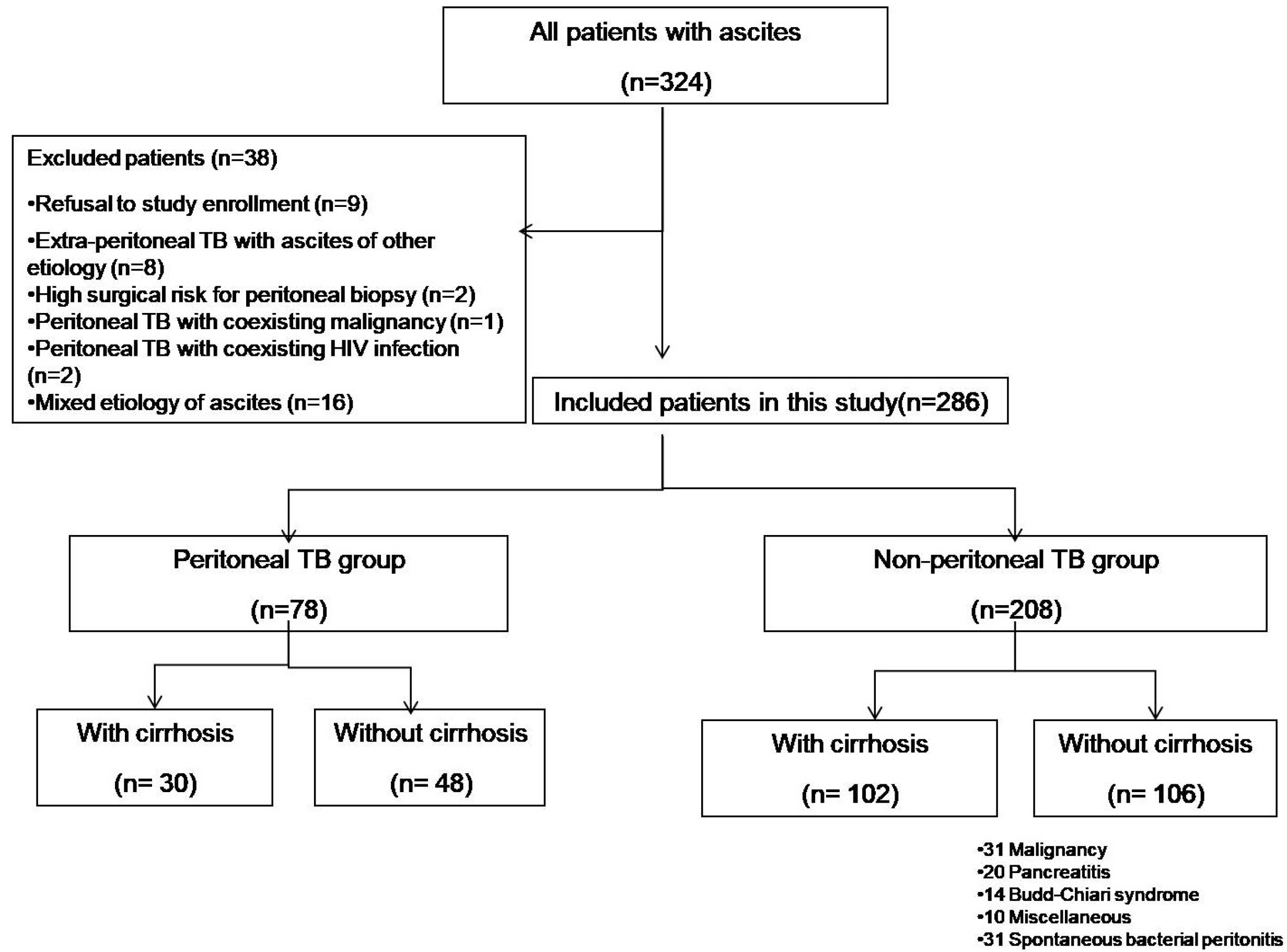
This study was a prospective observational case-control study that was conducted at the Department of Gastro- enterology, G.B. Pant Hospital, New Delhi, from October 2013 to December 2015. Prior approval for the study was obtained from the institutional ethics committee (IRB No. 11/IEC/MAMC/2011).
The study was designed to find the accuracy of ascitic fluid ADA for the diagnosis of peritoneal TB against the pathological diagnosis based on peritoneal biopsy. Consecutive patients ≥18 years in age presenting with new onset of ascites were evaluated. After a complete diagnostic evaluation, the patients were categorized as peritoneal TB and non-peritoneal TB group with or without cirrhosis. Cirrhosis was diagnosed based on the clinical, radiological, or pathological findings. Patients with coexistent tuberculosis at any other body site or possible mixed ascites from two etiologies were excluded.
2. Evaluation for the cause of ascites
All patients with ascites were evaluated for the etiology as per standard protocol. A complete blood count, liver function tests, kidney function tests, chest X-ray, abdominal ultrasonography, and ascitic fluid analysis, including cell count, albumin, protein, ADA, Gram, and AFB stain, was done in all subjects. The upper gastrointestinal endoscopy, colonoscopy, and computer-enhanced tomography of the abdomen and chest were performed on a case-to-case basis.
3. Measurement of ADA
ADA analysis was performed on freshly collected AF samples within 2 hours of collection of fluid. Ascitic Fluid ADA was determined by commercially available kits. We used BioSystems S.A. Costa Brava, 30.08030 Barcelona (Spain) (Quality System certified according to EN ISO 13485 and EN ISO 9001 standards) kit which uses Slaats Method for ADA determination.
4. Confirmation of peritoneal TB
A peritoneal biopsy was performed on patients in whom peritoneal TB was suspected after a clinical evaluation and baseline investigations, and in other cases, wherever required clinically. Most of these cases were suspected of malignant ascites and miscellaneous categories of rare etiologies with negative routine evaluations. Peritoneal TB was suspected in patients with a low serum ascites albumin gradient (SAAG), high protein, and clinical signs, such as fever, loss of appetite, loss of weight, the recent development of ascites. For peritoneal TB in cirrhotics, the same criteria were applied except for the SAAG and total protein. A peritoneal biopsy was conducted primarily laparoscopically. This was also done under ultrasound guidance in few patients wherever feasible. The visual findings during laparoscopy were noted, and peritoneal biopsy samples were sent for histopathological analysis. The definitive diagnosis of peritoneal TB was considered if a peritoneal tissue biopsy showed any of the following
25: 1) positive AFB stain, 2) caseating granuloma, and 3) Multiple large noncaseating epithelioid granulomas with Langhans giant cell with a high clinical suspicion with a response to antituberculosis drugs. All other patients who did not present the pathological findings of TB in peritoneal biopsy or who did not show the clinical findings of TB and skip peritoneal biopsy were referred to as the non-peritoneal TB group.
5. Statistical Analysis
The continuous data were expressed as the mean with standard deviation or 95% CI. The groups were compared using an independent Student t-test in the case of continuous data, while categorical data were compared using a Chi-Square test. The sensitivity, specificity, diagnostic accuracy, and area under the receiver operating characteristic curve (AUROC) were calculated for ADA for diagnosing peritoneal TB confirmed by peritoneal biopsy. Statistical Products and Services Solution (SPSS) software version 23.0 (SPSS, Chicago, IL, USA) was used for statistical analysis. For all statistical tests, a two-sided p-value <0.05 was considered significant.
DISCUSSION
Peritoneal TB had very high mortality of approximately 50% in the past, but improvements in diagnostic techniques and anti-TB medication decreased the mortality with the survival rate of almost 91%.
8,27,28 One of the important risk factors for peritoneal TB was cirrhosis as in the present study, in which 38% of the peritoneal TB patients had underlying cirrhosis. The clinical presentation was diverse, and in patients with cirrhosis, it may not be suspected initially, leading to a delay in the diagnosis and consequently the start of treatment.
9
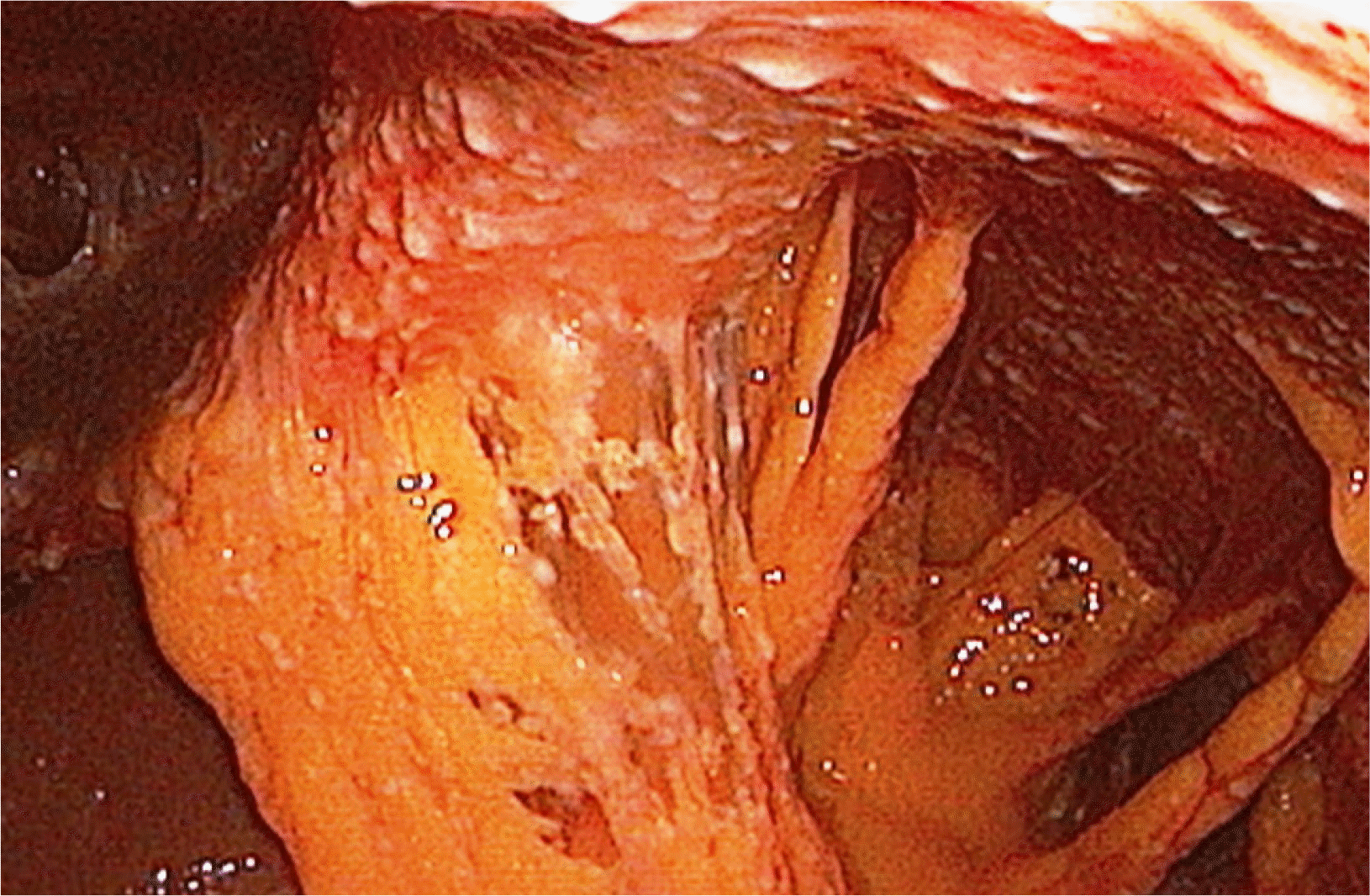
Laparoscopy is the diagnostic tool of choice because it provides a direct examination of the peritoneum, and one can take biopsies for histopathology and microbiological examination. On the other hand, it was associated with morbidity and mortality, even higher in cirrhotic patients.
13-16 It is also not practical for patients with severe underlying comorbidities to undergo laparoscopy. Therefore, patients with cirrhosis will often be poor candidates for laparoscopy. Ultrasound is an excellent non-invasive method for an evaluation of the intra-peritoneal cavity, and one can also take peritoneal biopsies.
29,30 On the other hand, ultrasound-guided biopsies require expertise and are not feasible for all patients. We were able to do laparoscopy in 47 patients while ultrasound guided biopsies were performed only on 31 patients (39.74%) (
Table 3;
Fig. 6).
The AFB staining of ascitic fluid has very poor sensitivity, and cultures also take a very long time.
9 This is quite a time-consuming process, even if a laparoscopic approach is followed. Good, less invasive, easy to perform tests with good sensitivity and specificity are needed because patients with a delayed diagnosis have higher mortality.
6
ADA has been studied in body fluids, including ascitic fluid for diagnosing TB, and has been shown to have high sensitivity and specificity. ADA is an essential enzyme in the purine salvage pathway; it plays an important role in T cell differentiation. Thus far, three meta-analyses have been published dealing with the role of ADA in peritoneal TB diagnosis.
17-19 Unfortunately, ADA for diagnosis of peritoneal TB in patients with cirrhosis has been studied.
20-24 Hillenbrand et al. found that the ADA is inferior in cirrhosis as a diagnostic method for peritoneal TB. In this study, the sensitivity of ADA was only 30% for diagnosing peritoneal TB in patients with cirrhosis.
23 They speculated that this could be because ADA correlates positively with the ascitic fluid total protein, and cirrhotic patients generally have low ascitic fluid total protein. The major drawbacks of this study were the small sample size (n=10), the method of ADA analysis used was different, and the ADA estimation was done on stored samples.
23 On the other hand, recent studies revealed high sensitivity (94-100%) and high specificity (92-93%) of ADA in diagnosing peritoneal TB in patients with cirrhosis.
20-22,24 On the other hand, these studies also had a small sample size (all four studies altogether had 29 patients) with a small or no control group.
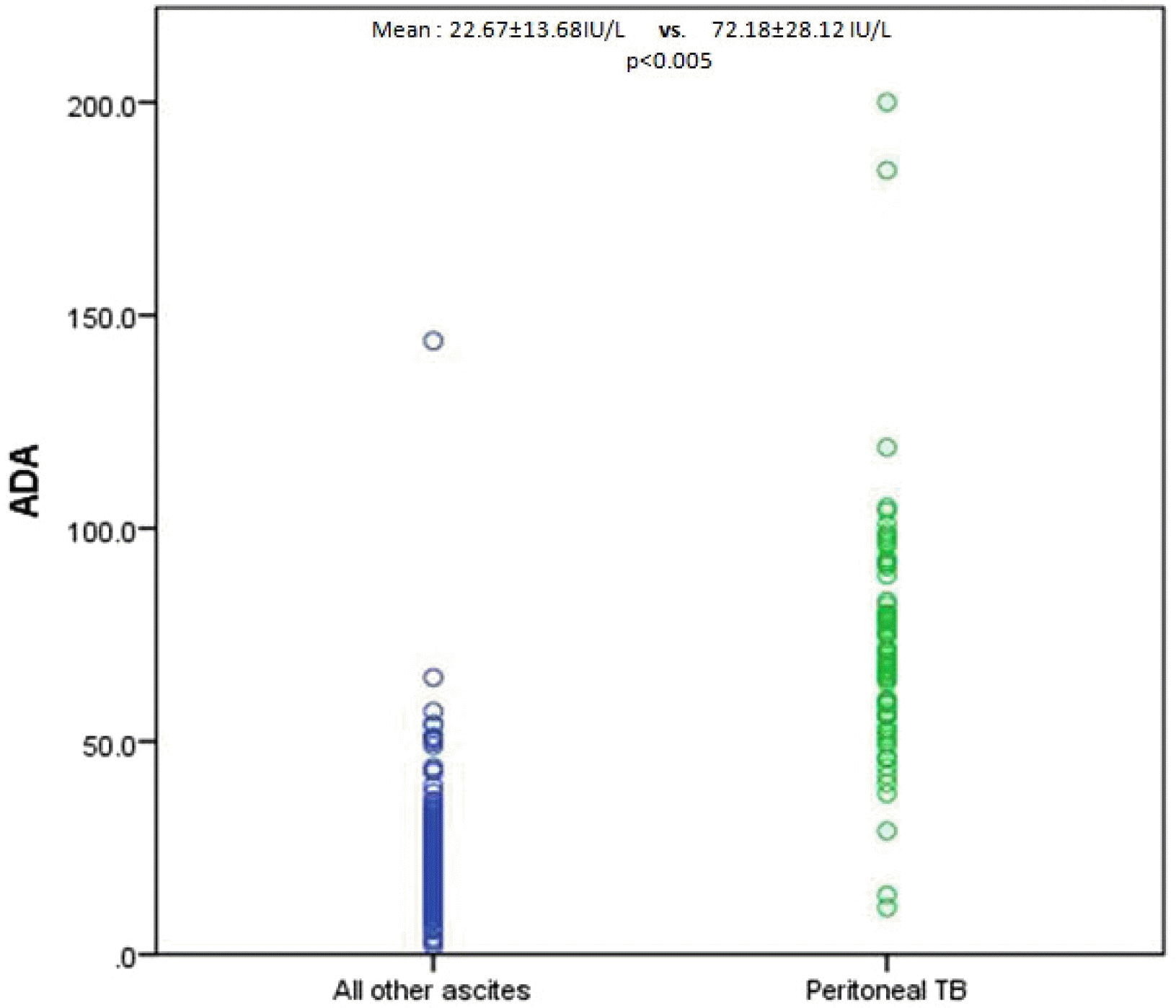
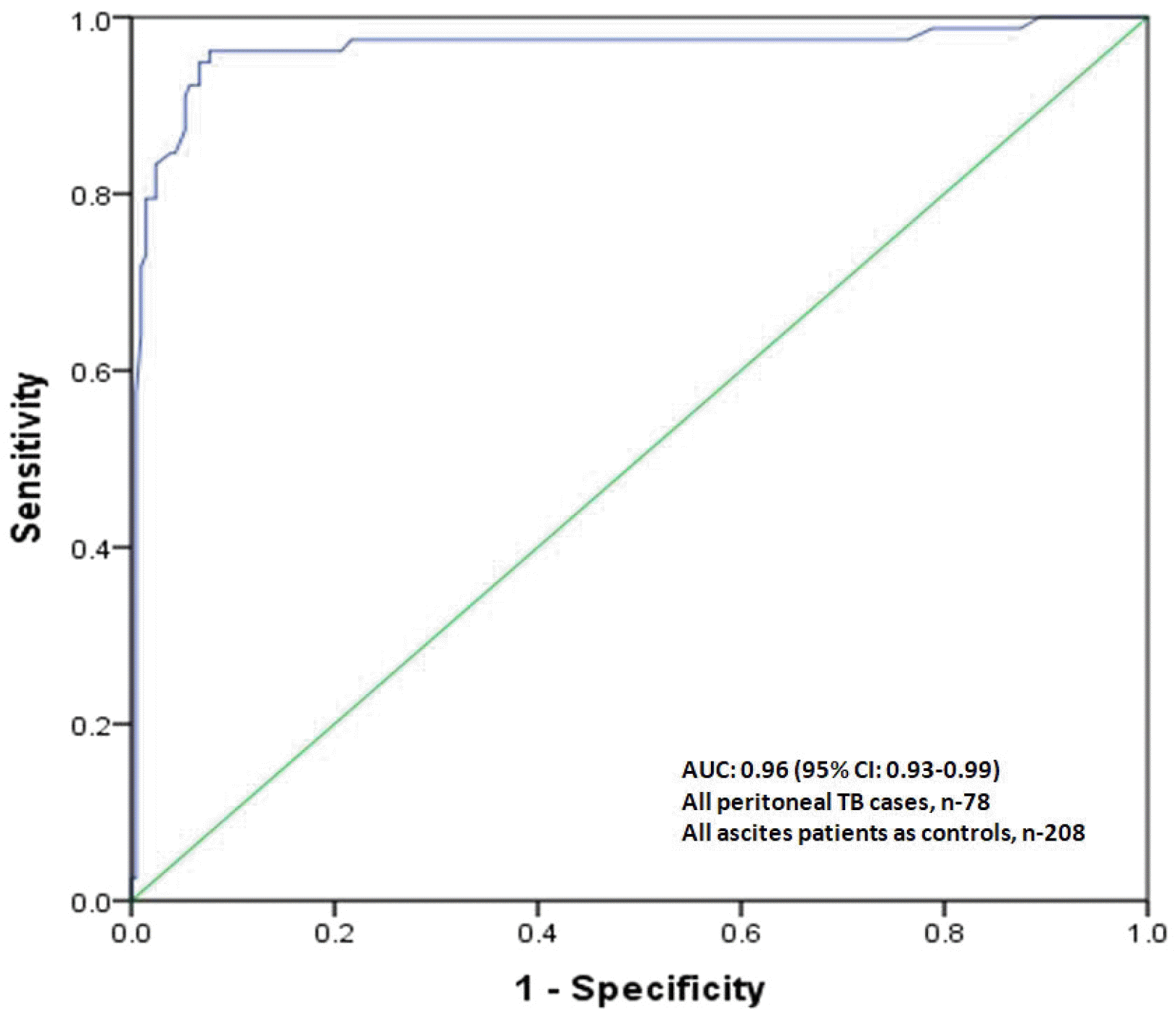
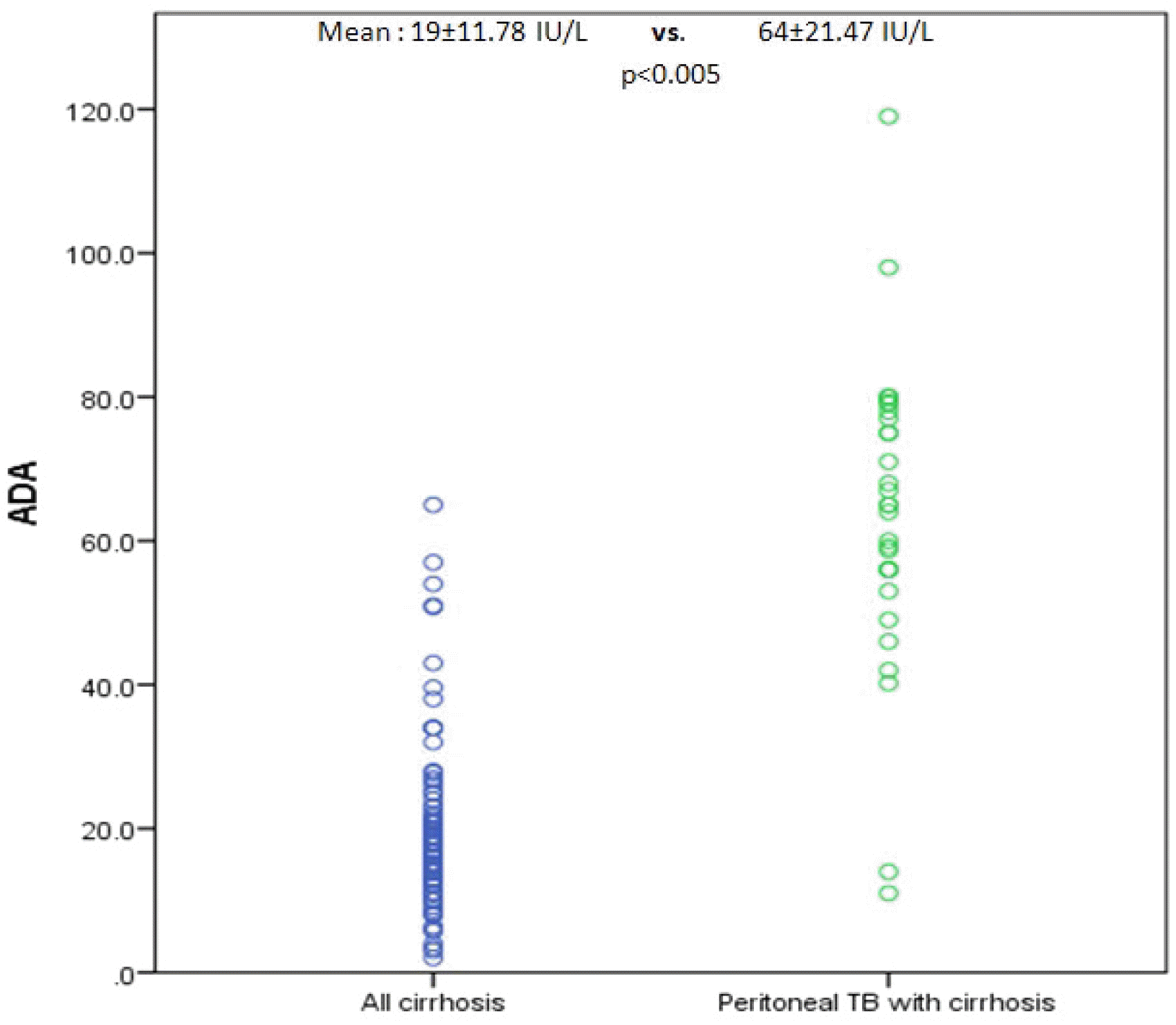
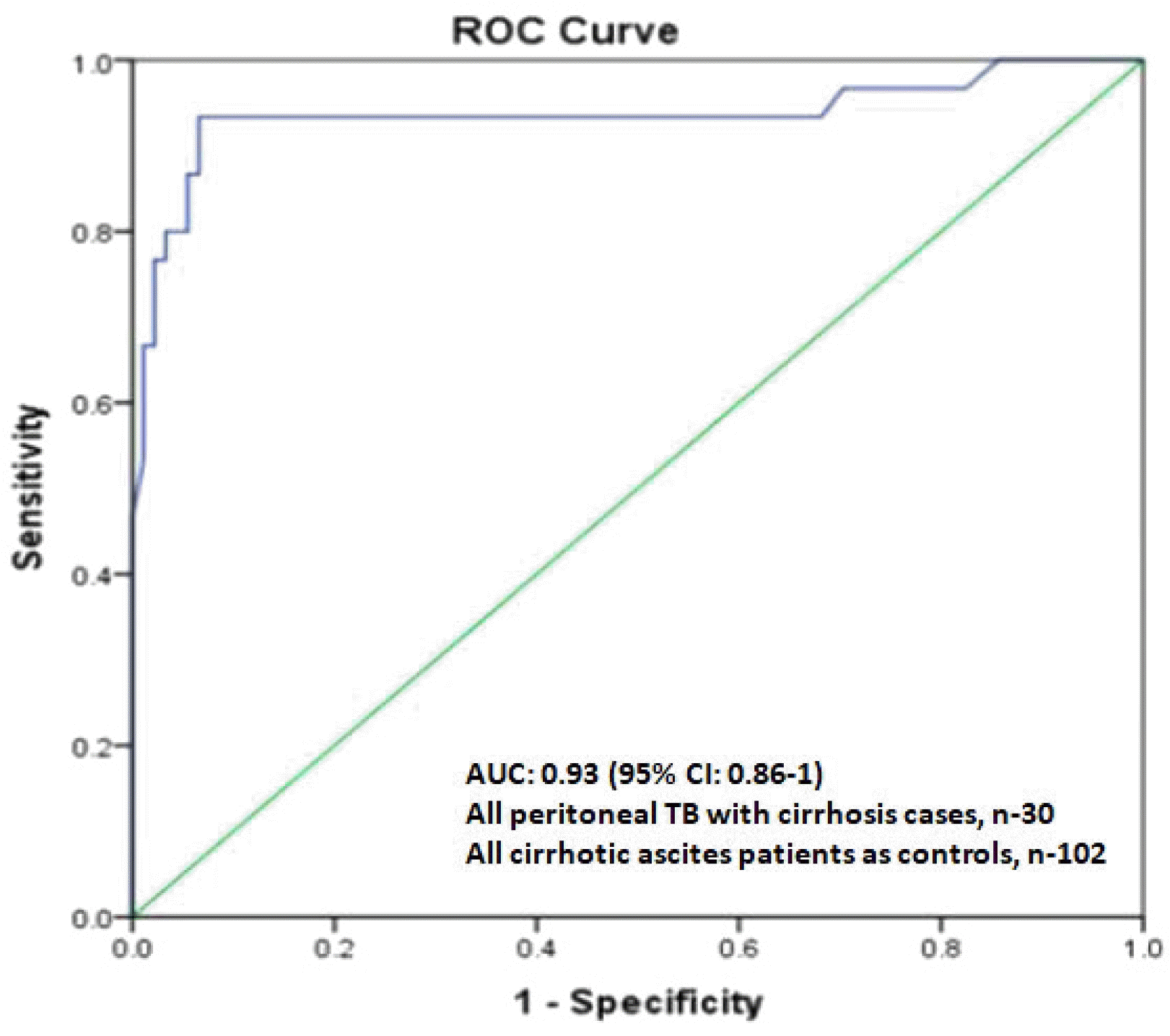
In the present study, ADA overall had very high sensitivity and specificity at a cutoff of 41.1 IU/L. Furthermore, ADA had sensitivity, specificity, and accuracy of 93%, 94%, and 92%, respectively, for diagnosing peritoneal TB in cirrhotic patients at a slightly lower cutoff of 39.9 IU/L compared to non-cirrhotic patients. Therefore, the ADA levels in the cirrhotic peritoneal TB group were much higher than the cirrhotic non-peritoneal group and all other types of ascites patients in the control group.
In the present study, ascitic fluid ADA correlated well with the ascitic fluid total protein (r2 =0.62, p=<0.005). The ADA levels in the cirrhotic peritoneal TB group were much higher despite having a low ascitic fluid total protein than the non-cirrhotic peritoneal TB group. In addition, high total protein does not mean high ADA levels because it can be observed in patients with pancreatic ascites and malignant ascites, where the ascitic fluid total protein was high in all but ADA. Therefore, some other factors act for the increase in ADA in the ascitic fluid in the presence of peritoneal TB.
The ADA has false positivity in 6.9-9.4% ascitic fluids, as depicted in published studies.
20,23 In the present study, 14 patients (6.7%) in the control group had an ADA value ≥40 IU/L, including ascitic patients with SBP, pancreatic ascites, malignant ascites, and lymphatic obstruction with ascites. In the SBP patients, post-treatment ascitic fluid ADA was repeated at four weeks and was below the cutoff. To deduce any firm opinion from this observation is difficult, but a repeat ADA measurement after treating the obvious clinical problem when the clinical scenario does not fit might help clinicians. In this study, only four patients in the peritoneal TB groups had ADA <40 IU/L, two patients were already on anti-TB medication, and while the other two patients had neutrophilic dominant ascites (>80% neutrophils in ascitic fluid). Anti-TB medication and low lymphocyte count in the ascitic fluid may have resulted in a lower ADA value in these patients.
The present study is the largest single-center study in terms of the number of patients and the patients with cirrhosis and peritoneal TB. Moreover, a large control with a heterogeneous population makes this study clinically robust. The gold standard in the present study was histopathology on peritoneal tissue, which is highly reliable and accurate (
Table 4).
This study had a few limitations. First, the peritoneal biopsy was not applied as the gold standard diagnostic tool for peritoneal TB to all patients with ascites. Instead, the suspected group was selected for peritoneal TB, and the peritoneal biopsy was performed. Therefore, there could be false-negative cases among the non-peritoneal TB group. To the best of the authors’ knowledge, there was no overt peritoneal TB case at least within one month after the initial assessment. In addition, the diagnostic approach might avoid the ethical issues associated with a non-selective biopsy on many non-peritoneal TB patients, as well as the potential complications without clinical benefit. Second, the patients with ascites with mixed causes were excluded from making the diagnostic meaning of ADA clear-cut. Therefore, it is unclear if the ascitic ADA level is useful in these patients. Third, this result is based on the data from a single center in a country. Therefore, care should be taken to apply the findings to other populations.
In conclusion, when a patient visits a clinic for ascites, ascitic ADA measurements in the cases of suspicion of tuberculosis might show excellent diagnostic accuracy for peritoneal TB in non-cirrhotic and cirrhotic patients. Further studies will be needed to delineate the meaning of ADA level in the patients with ascites of mixed causes.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download