Abstract
Background
Allogeneic hematopoietic stem cell transplantation (alloSCT) is a potentially curative treatment option for acute leukemia. We aimed to identify the comorbidity factors affecting survival outcomes after alloSCT and develop a new comorbidity index tool for predicting overall survival (OS).
Methods
A Korean nationwide cohort of 3,809 adults with acute leukemia treated with alloSCT between January 2002 and December 2018 was analyzed as the development cohort. A retrospective cohort comprising 313 consecutive adults with acute leukemia who underwent alloSCT between January 2019 and April 2020 was analyzed as the validation cohort.
Results
In the development cohort, advanced age, male sex, and comorbidities such as previous non-hematologic malignancy, hypertension, and coronary or cerebral vascular disease were significantly related to poor OS. Subsequently, a new comorbidity scoring system was developed, and risk groups were created, which included the low-risk (score ≤0.17), intermediate-risk (0.17< score ≤0.4), high-risk (0.4< score ≤0.55), and very high-risk (score >0.55) groups. The 1-year OS rates were discriminatively estimated at 73.5%, 66.2%, 61.9%, and 50.9% in the low-risk, intermediate-risk, high-risk, and very high-risk groups in the development cohort, respectively (P<0.001). The developed scoring system yielded discriminatively different 1-year OS rates and 1-year incidence of non-relapse mortality according to the risk group (P=0.085 and P=0.018, respectively). Furthermore, the developed model showed an acceptable performance for predicting 1-year non-relapse mortality with an area under the curve of 0.715.
Although novel therapies have recently been introduced [1, 2], allogeneic hematopoietic stem cell transplantation (alloSCT) is still regarded as the only curative modality for acute leukemia [3-6]. However, a low overall survival (OS) related to relapse or treatment-related mortality is an important obstacle that compromises the efficacy of alloSCT [7]. Thus, a precise risk-adapted approach remains an unmet need in clinical practice.
In addition to emerging biological factors (such as adverse cytogenetics, failure to achieve minimal residual disease, and intolerance to chemotherapeutic toxicities), the comorbidity of individuals could have tremendous impacts on the prognosis of alloSCT. In this regard, various models, including the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) [8], comorbidity-age index [9], and Charlson comorbidity index [10], have been used for pre-transplant risk stratification prior to alloSCT based on the patient’s comorbidities. These indices also provide a well-defined risk stratification for mortality. Nevertheless, these indices were not established in a nationwide cohort of patients with acute leukemia. Furthermore, the prognostic impact of commonly emphasized variables among the aforementioned comorbidity indices, such as diabetes, hypertension, cerebrovascular disease, pulmonary dysfunction, and prior non-hematologic malignancy, has not yet been validated in a Korean nationwide cohort.
In Korea, the Korean National Health Insurance Service (KNHIS) program is a mandatory public health insurance system that covers approximately 98% of the overall Korean population. The KNHIS database contains universal medical claims and mortality for the entire Korean population [11] and has been used in various epidemiological research studies on hematologic diseases, as described in detail elsewhere [12-15]. These strengths enable research on a nationwide cohort with the endpoint of identifying personal comorbidities related to patient prognosis after alloSCT.
Accordingly, this study aimed to verify whether well-known patient comorbidity-related prognostic factors, including age, sex, previous non-hematologic malignancy, hypertension, diabetes, dyslipidemia, chronic obstructive pulmonary disease (COPD), cerebrovascular or cardiovascular disease (CVA), anxiety disorder, and depression, have prognostic impacts on outcomes of alloSCT using KNHIS data. We were also interested in the development of a prognostic scoring system based on identified individual comorbidities, followed by external validation of the developed system using an independent database of the development cohort from the KNHIS data.
Data for the development cohort were extracted from the KNHIS database. We first included adult patients (≥18 yr) who underwent alloSCT between January 2002 and December 2018 in the KNHIS database using the procedure codes V073, X5061, and/or X5063. These claims codes represent the performance of alloSCT, the collection of bone marrow stem cells, and the collection of mobilized peripheral blood stem cells. Thereafter, we confirmed that the final cohort comprised cases of acute leukemia classified based on the International Classification of Diseases, Tenth Revision (ICD-10) codes C92, C93, and/or C94 for acute myeloid leukemia, C91 for acute lymphoblastic leukemia, and/or C95 for unclassified acute leukemia. The requirement for individual patient consent was waived because of the anonymous nature and public availability of the data.
Data from consecutive adult patients with acute leukemia who underwent alloSCT between January 2019 and April 2020 at the Catholic Hematologic Hospital were used to construct the validation cohort. Data were collected until April 2021 to have at least a 1-year follow-up period. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital, Seoul, Korea (KC19ZNSI0396) and was conducted in accordance with the Declaration of Helsinki.
For individuals treated with multiple alloSCTs, the baseline date for calculating OS was defined as the date of the first alloSCT. We used the ICD-10 codes to define whether the patient presented with comorbidities, including previous non-hematologic malignancy (ICD-10 code: Cxx, except for C83, C86, C90, C91, C92, C93, C94, and/or C95 indicating hematologic malignancies), hypertension (ICD-10 code: I10, I11, I12, I13, I14, and/or I15), diabetes (ICD-10 code: E10, E11, E12, E13, and/or E14), dyslipidemia (ICD-10 code: E78), chronic obstructive pulmonary disease (COPD; ICD-10 code: J44), anxiety disorder (ICD-10: F30), and depression (ICD-10: F32 and/or F33). CVA was indicated when the patient was administered antiplatelet agents (drug codes: 117101ATB, 136901ATB, 157702ACR, 162403ATR, 165001ACH, 194930ATB, 492501ATB, 495201ATB, 498801ATB, 498900ATB, 501501ATB, 517900ACE, 517900ATE, 597301ATB, 597302ATB, 615901ATB, 615902ATB, 659501BIJ, and/or 667500ACE) and/or anticoagulants (drug codes: 511401ATB, 511403ATB, 511402ATB, 511404ATB, 249103ATB, 249105ATB, 613701ACH, 613702ACH, 617001ATB, 617002ATB, 643602ATB, 643601ATB, and/or 643603ATB).
In the development cohort, all risk factors potentially related to OS with a P-value of <0.05 in the univariable analysis were entered into the multivariable model to confirm the factors associated with OS. Multivariable analysis was performed using the Cox proportional hazards regression model. The final parameters used in the scoring system were defined by a P-value of <0.05 in the final multivariable model. The risk score for each significant parameter was assigned by adjusting the hazard ratio (HR) values to a loge scale.
Using the scoring system created using the development cohort, we performed an analysis to validate whether the scoring system performed well as a reliable prognostic tool in the validation cohort. OS and the incidence of non-relapse mortality (NRM) and relapse were compared between the risk groups. Finally, discriminatory performance was assessed by receiver operating characteristic (ROC) curve analysis.
Numerical variables not exhibiting a normal distribution are presented as medians (range, minimum–maximum). Categorical variables are presented as numbers (%). OS was defined as the time from the date of alloSCT to death (from any cause) or the date of the last follow-up. OS rates at 1 year were calculated using the Kaplan-Meier method and compared using the log-rank test. We calculated the NRM probability and relapse rates using cumulative incidence estimation based on the competing risks of relapse and NRM. The area under the curve (AUC) of the ROC curve was calculated to predict the accuracy of the validation cohort analysis. An AUC value >0.7 was considered reliable [16]. DeLong’s test was used to compare the statistical differences between the AUCs. All statistical analyses were conducted using R statistical software (ver. 3.6.1, R Foundation for Statistical Computing, Vienna, Austria, 2019). Statistical significance was set at P<0.05.
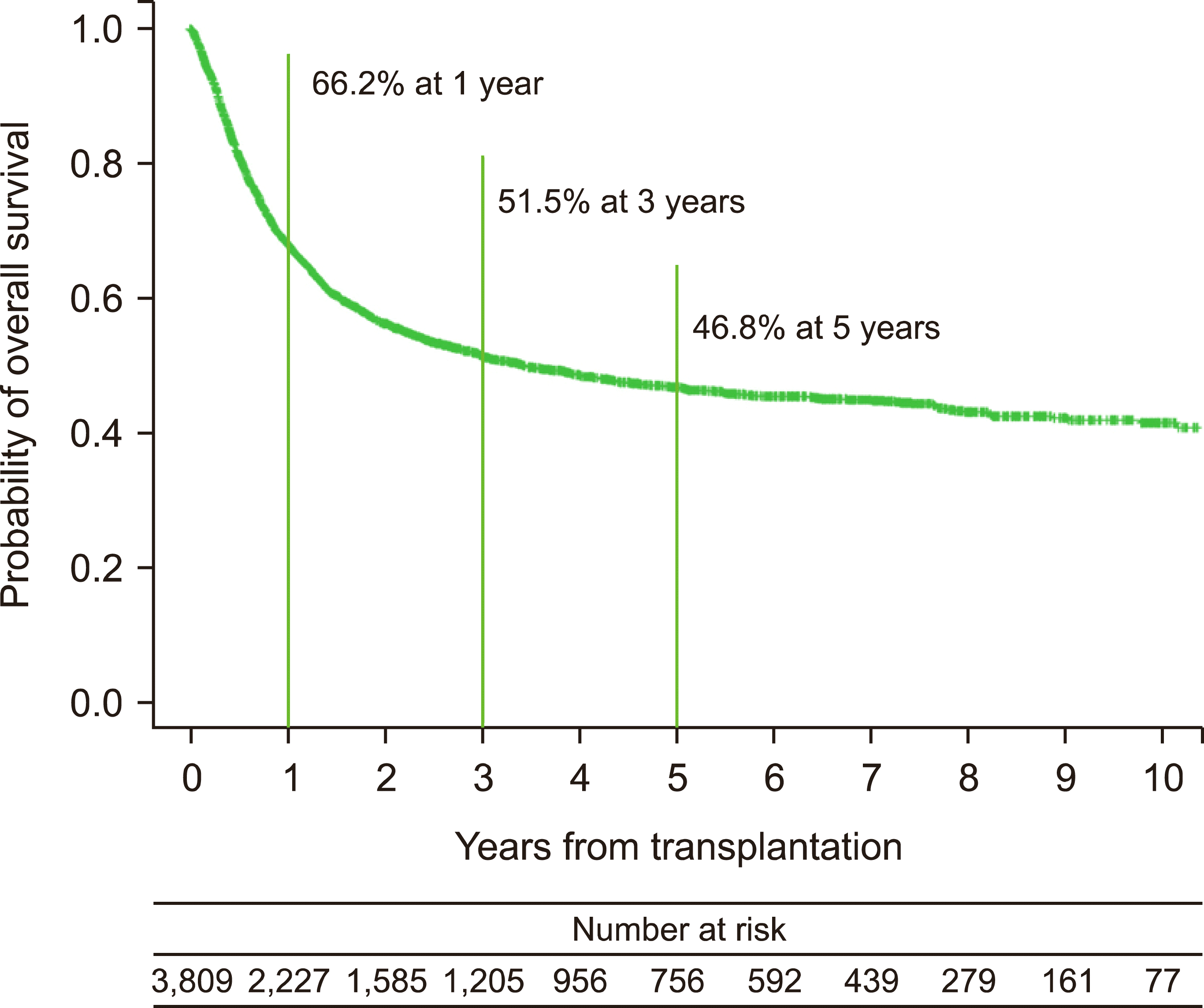
Development cohort: In total, 8,230 patients who underwent alloSCT between January 2002 and December 2018 were identified. Patients aged <18 years (N=1,060) and those having other hematologic diseases (N=3,361) were excluded from the analysis. Accordingly, 3,809 patients with acute leukemia were included in the development cohort (Fig. 1A). The baseline demographics are summarized in Table 1. Overall, the median age of the patients was 47 years (range, 18–74 yr), and 54.0% of the patients were males (N=2,055). Regarding the underlying comorbidities at baseline, previous non-hematologic malignancy, hypertension, diabetes, dyslipidemia, COPD, CVA, anxiety disorder, and depression were present in 387 (10.2%), 1,224 (32.1%), 1,125 (29.5%), 2,135 (56.1%), 191 (5.0%), 166 (4.4%), 900 (23.6%), and 613 (16.1%) patients, respectively. With a median follow-up of 50.2 months [95% confidence interval (CI), 47.7–53.2], the 1-year, 3-year, and 5-year OS rates were estimated as 68.2% (95% CI, 66.7–69.7), 51.5% (95% CI, 49.8–53.2), and 46.8% (95% CI, 45.0–48.6) in the total cohort, respectively (Fig. 2).
Validation cohort: Data on 576 consecutive patients treated with alloSCT were collected. Following the exclusion of patients aged <18 years (N=128) and those having other hematologic diseases (N=135), a validation cohort of 313 patients was eligible for analysis (Fig. 1B). The baseline characteristics of the validation cohort are shown in Table 1.
The univariable analysis identified the following potential factors related to poor OS: age (≥65 years or 50–64 years compared with <50 years); male sex; comorbidities such as previous non-hematologic malignancy, hypertension, diabetes, dyslipidemia, CVA, and anxiety disorder/depression. In the multivariable analysis, we confirmed that 5 variables, including advanced age (50–64 years or ≥65 years), male sex, non-hematologic malignancy, hypertension, and CVA, were significantly associated with poor OS, as shown in Table 2.
Based on the multivariable analysis, an assigned risk score of each variable, obtained by the log-scale of the HR (Table 3) are as follows: age (50–64 years, 0.21 points; ≥65 years, 0.55 points), male sex (0.13 points), previous non-hematologic malignancy (0.17 points), hypertension (0.13 points), and CVA (0.4 points). The risk score was computed as the sum of each variable score, resulting in a median risk score of 0.2 and a range of 0–1.38. We then classified the patients into 10 subgroups, whose ranks were determined by the order of the decile risk scores. Based on the OS rates according to these 10 subgroups (Fig. 3A), the risk score was finally stratified into 4 risk groups (Fig. 3B): the 1-year OS/5-year OS rates were 73.5% [(95% CI, 71.4–75.6)/52.9% (95% CI, 50.4–55.6)], 66.2% [(95% CI, 63.6–68.9)/44.0% (95% CI, 41.0–47.2)], 61.9% [(95% CI, 57.2–67.0)/37.3% (95% CI, 31.9–43.6)], and 50.9% [(95% CI, 44.8–57.7)/29.6% (95% CI, 23.4–37.5)] in the low-risk (score ≤0.17), intermediate-risk (0.17< score ≤0.4), high-risk (0.4< score ≤0.55), and very high-risk (score >0.55) groups, respectively. The developed scoring system calculator is presented in the Supplementary calculator.
With a median follow-up of 18.8 months (95% CI, 18.1–20.0) in the validation cohort, the 1-year OS rate and 1-year cumulative incidence of NRM and relapse were 74.4% (95% CI, 69.2–78.9), 11.5% (8.3–15.3), and 23.0% (18.5–27.8), respectively (Supplementary Fig. 1). When the developed scoring system was applied to the validation cohort, the 1-year OS rates were measured differently according to the risk group (P=0.085), which were 79.4% (95% CI, 72.2–84.9) in the low-risk group, 74.2% (95% CI, 64.3–81.8) in the intermediate-risk group, 60.0% (95% CI, 35.7–77.6) in the high-risk group, and 61.1% (95% CI, 35.7–77.6) in the very high-risk group. There were significant differences in both the 1-year OS rate between a combined group of the low-risk and intermediate groups and another combined group of the high-risk and very high-risk groups at 77.4% (95% CI, 71.8–82.1) and 60.7% (95% CI, 46.7–72.1), respectively (P=0.018) (Fig. 4A).
The 1-year cumulative incidence of NRM was significantly different according to the risk groups (P=0.035), which was 7.5% (95% CI, 4.1–12.3) in the low-risk group, 11.3% (95% CI, 6.0–18.6) in the intermediate-risk group, 20.0% (95% CI, 5.9–40.0) in the high-risk group, and 25.0% (95% CI, 12.2–40.0) in the very high-risk group (Fig. 4B). However, we observed no significant difference in the 1-year cumulative incidence of relapse according to the risk group (P=0.349, Fig. 4C). In the ROC curve analysis, the developed scoring system achieved an AUC of 0.715 (95% CI, 0.658–0.772), indicating reliable discrimination of NRM events at 1 year in the validation cohort (Fig. 4D).
This study developed a new scoring system to predict patient prognosis after alloSCT in 3,809 patients with acute leukemia using a development cohort derived from a nationwide database. With a 5-year OS probability of 46.8% in the development cohort, we created a comorbidity index scoring system comprising age, sex, previous non-hematologic malignancy, hypertension, and CVA. The risk score was significantly stratified into 4 risk groups: low-risk, intermediate-risk, high-risk, and very high-risk groups associated with 1-year/5-year OS rate probabilities of 73.5%/52.9%, 66.2%/44%, 61.9%/37.3%, and 50.9%/29.6%, respectively. The validation cohort analysis indicated that the developed comorbidity index scoring system was statistically feasible for predicting OS and NRM. AlloSCT is generally planned as a consolidative procedure following intensive chemotherapy in patients who achieve complete remission after intensive chemotherapy. In the validation cohort analysis, we observed interesting findings that there were significant increasing trends in the proportion of patients presenting with comorbidities at pre-alloSCT compared with the diagnosis of their disease (Supplementary Fig. 2). Therefore, our results suggest that updated comorbidity profiles at the time of alloSCT, as well as the diagnosis of acute leukemia, should be monitored rigorously because complications following intensive chemotherapy could indicate a new comorbidity, although it was absent at the time of diagnosis.
In the validation cohort of the current study, we evaluated the feasibility of the developed scoring system, which provided discriminative predictability for OS and NRM. In addition to our system, HCT-CI, which comprises 17 different categories of organ dysfunction [8], has not only been used as the most reliable tool for the comorbidity-based risk assessment of survival and NRM after alloSCT but has also been successfully validated in transplant institutions worldwide [17-19]. However, the cohort size of the original study (N=1,055) for the establishment of HCT-CI, as well as studies of validation (the largest among all studies to the best of our knowledge, N=324), was relatively small compared with our study. Moreover, multi-institutional validation of the predictive power of HCT-CI has not yet been documented. Interestingly, a multicenter prospective study by a Japanese group found that HCT-CI failed to predict NRM [20]. Therefore, there is an unmet need for better tools to optimize comorbidities based on risk stratification for alloSCT. Although our developed comorbidity index model should be further validated in a future study, the supplemental analysis showed that our new index demonstrated better 1-year NRM prediction than the HCT-CI system in the validation cohort, with an AUC of 0.688 vs. 0.509, P<0.001 (Supplementary Fig. 3). This result also illustrated an AUC of 0.509 for HCT-CI in predicting 1-year NRM, which is not discriminated, as in the abovementioned results from the multicenter Japanese prospective study.
Compared with prior studies depicting risk stratification according to comorbidities, our results had several strengths. First, to the best of our knowledge, the risk factors for survival were analyzed using a nationwide cohort comprising the largest number of patients from a Korean multicenter cohort. Second, unlike the aforementioned scoring systems that require endoscopy, echocardiography, pulmonary function tests, and various laboratory tests to generate the results, our scoring system was created using easily accessible data such as age and underlying comorbidities. Third, despite the small number of variables comprising the scoring system, we believe that the discriminatory power, as well as the validity of our system for predicting survival in the validation cohort, which was distinctively independent of the development cohort, appeared to be reliable.
There are several limitations to our study. In the development cohort analysis, we were unable to investigate all variables comprising the HCT-CI because the KNHIS database has the inherent limitation that concrete results derived from laboratory tests, echocardiography, and pulmonary function tests were absent. In this regard, the severity of the comorbidity could not be assessed. Second, due to unreliable data related to the cause of death in the KNHIS data, the cumulative incidence of relapse-related death or NRM was not explored in the development cohort analysis. Third, this study could be limited by the relatively small size, short-term follow-up, and retrospective design of the validation cohort. Fourth, we believe that the results of the current study cannot be generalized because other important factors, including characteristics of acute leukemia and treatment factors, were not entered as covariants to verify the prognostic impact of patient-related comorbidities.
Despite these limitations, our study provides a feasible scoring system based on comorbidity information that is easy to obtain for predicting survival prognosis among patients with acute leukemia who undergo alloSCT. Therefore, the current results could be attributed to better clinical decision-making based on patient-driven risk-adaptive strategies for alloSCT.
ACKNOWLEDGMENTS
The authors acknowledge the efforts of the staff of the Health Insurance Review Health Insurance Review and Assessment Service, which is supported by the Korean National Health Insurance Service (KNHIS). We thank Si-Hyun Park and Seunghoon Han for their help in analyzing the KNHIS data. This work was supported by the Research Foundation of Internal Medicine, Catholic University of Korea.
REFERENCES
1. Mukherjee S, Sekeres MA. 2019; Novel therapies in acute myeloid leukemia. Semin Oncol Nurs. 35:150955. DOI: 10.1016/j.soncn.2019.150955. PMID: 31759818.

2. Samra B, Jabbour E, Ravandi F, Kantarjian H, Short NJ. 2020; Evolving therapy of adult acute lymphoblastic leukemia: state-of-the-art treatment and future directions. J Hematol Oncol. 13:70. DOI: 10.1186/s13045-020-00905-2. PMID: 32503572. PMCID: PMC7275444.

3. Tallman MS, Wang ES, Altman JK, et al. 2019; Acute myeloid leukemia, version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 17:721–49. DOI: 10.6004/jnccn.2019.0028. PMID: 31200351.
4. Yoon JH, Kim HJ, Park SS, et al. 2017; Long-term clinical outcomes of hematopoietic cell transplantation for intermediate-to-poor-risk acute myeloid leukemia during first remission according to available donor types. Oncotarget. 8:41590–604. DOI: 10.18632/oncotarget.15295. PMID: 28206975. PMCID: PMC5522252.

5. Yoon JH, Yhim HY, Kwak JY, et al. 2016; Minimal residual disease-based effect and long-term outcome of first-line dasatinib combined with chemotherapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann Oncol. 27:1081–8. DOI: 10.1093/annonc/mdw123. PMID: 26951627.

6. Brown PA, Wieduwilt M, Logan A, et al. 2019; Guidelines insights: acute lymphoblastic leukemia, version 1.2019. J Natl Compr Canc Netw. 17:414–23. DOI: 10.6004/jnccn.2019.0024. PMID: 31085755.
7. Bacigalupo A, Sormani MP, Lamparelli T, et al. 2004; Reducing transplant-related mortality after allogeneic hematopoietic stem cell transplantation. Haematologica. 89:1238–47. PMID: 15477210.
8. Sorror ML, Maris MB, Storb R, et al. 2005; Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 106:2912–9. DOI: 10.1182/blood-2005-05-2004. PMID: 15994282. PMCID: PMC1895304.

9. Sorror ML, Storb RF, Sandmaier BM, et al. 2014; Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 32:3249–56. DOI: 10.1200/JCO.2013.53.8157. PMID: 25154831. PMCID: PMC4178523.

10. Charlson M, Szatrowski TP, Peterson J, Gold J. 1994; Validation of a combined comorbidity index. J Clin Epidemiol. 47:1245–51. DOI: 10.1016/0895-4356(94)90129-5. PMID: 7722560.

11. Kim DS. 2010; Introduction: health of the health care system in Korea. Soc Work Public Health. 25:127–41. DOI: 10.1080/19371910903070333. PMID: 20391257.

12. Wang SM, Park SS, Park SH, et al. 2020; Pre-transplant depression decreased overall survival of patients receiving allogeneic hematopoietic stem cell transplantation: a nationwide cohort study. Sci Rep. 10:15265. DOI: 10.1038/s41598-020-71208-2. PMID: 32943660. PMCID: PMC7499172.

13. Byun JM, Lee J, Shin SJ, Kang M, Yoon SS, Koh Y. 2018; Busulfan plus melphalan versus high-dose melphalan as conditioning regimens in autologous stem cell transplantation for newly diagnosed multiple myeloma. Blood Res. 53:105–9. DOI: 10.5045/br.2018.53.2.105. PMID: 29963515. PMCID: PMC6021568.

14. Kong SG, Jeong S, Lee S, Jeong JY, Kim DJ, Lee HS. 2021; Early transplantation-related mortality after allogeneic hematopoietic cell transplantation in patients with acute leukemia. BMC Cancer. 21:177. DOI: 10.1186/s12885-021-07897-3. PMID: 33602150. PMCID: PMC7891151.

15. Wang SM, Park SS, Park SH, et al. 2021; Pre-transplant dementia is associated with poor survival after hematopoietic stem cell transplantation: a nationwide cohort study with propensity score matched control. Clin Psychopharmacol Neurosci. 19:294–302. DOI: 10.9758/cpn.2021.19.2.294. PMID: 33888658. PMCID: PMC8077055.

16. Mandrekar JN. 2010; Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 5:1315–6. DOI: 10.1097/JTO.0b013e3181ec173d. PMID: 20736804.

17. Sorror ML, Giralt S, Sandmaier BM, et al. 2007; Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 110:4606–13. DOI: 10.1182/blood-2007-06-096966. PMID: 17873123. PMCID: PMC2234788.
18. Maruyama D, Fukuda T, Kato R, et al. 2007; Comparable antileukemia/lymphoma effects in nonremission patients undergoing allogeneic hematopoietic cell transplantation with a conventional cytoreductive or reduced-intensity regimen. Biol Blood Marrow Transplant. 13:932–41. DOI: 10.1016/j.bbmt.2007.04.004. PMID: 17640597.

19. Park SS, Jeon YW, Min GJ, et al. 2019; Graft-versus-host disease-free, relapse-free survival after allogeneic stem cell transplantation for myelodysplastic syndrome. Biol Blood Marrow Transplant. 25:63–72. DOI: 10.1016/j.bbmt.2018.08.004. PMID: 30103018.

20. Nakaya A, Mori T, Tanaka M, et al. 2014; Does the hematopoietic cell transplantation specific comorbidity index (HCT-CI) predict transplantation outcomes? A prospective multicenter validation study of the Kanto Study Group for Cell Therapy. Biol Blood Marrow Transplant. 20:1553–9. DOI: 10.1016/j.bbmt.2014.06.005. PMID: 25034961.

Fig. 1
Flow diagram of the con-struction of the development and validation cohorts.
Abbreviation: KNHIS, Korean National Health Insurance Service.
Fig. 3
Probability of overall survival (OS) according to (A) decile risk scores and (B) the final risk groups in the development cohort. Using decile risk scores, we classified the patients into 10 groups: rank 1 (score ≤0.17), rank 2 (score, >0.17 and ≤0.26), rank 3 (score, >0.26 and ≤0.4), rank 4 (score, >0.4 and ≤0.55), rank 5 (score, >0.55 and ≤0.68), rank 6 (score, >0.68 and ≤0.81), rank 7 (score, >0.81 and ≤0.91), rank 8 (score, >0.91 and ≤1.08), rank 9 (score, >1.08 and ≤1.21), and rank 10 (>1.21). Based on the 5-year OS rates in each rank group, we then stratified the patients into 4 risk groups. The low-risk group included patients with rank 1; the intermediate-risk group included patients with ranks 2 and 3; the high-risk group included patients with rank 4; and the very high-risk group included patients with ranks 5, 6, 7, 8, 9, and 10. The log-rank test showed significant differences in the OS among the risk groups (P<0.001).
Fig. 4
Validation of the developed scoring system in the validation cohort. (A) The 1-year overall survival (OS) rate was divided according to the risk groups (P=0.085). The post-hoc analysis illustrated a better 1-year OS rate in the low- or intermediate-risk groups than that in the high- or very high-risk groups (P=0.018, * is indicated in the Fig. 1A for the pos-hoc analysis). (B) The cumulative incidence of non-relapse mortality (NRM) was significantly divided according to the risk groups (P=0.035), (C) whereas the cumulative incidence of relapse was not significantly different between the 4 risk groups (P=0.349). (D) A receiver operating characteristic curve analysis achieved an area under the curve (AUC) of 0.715 (95% CI, 0.658–0.772) for predicting NRM events 1-year post-allogeneic hematopoietic stem cell transplantation.
Table 1
Demographics of the cohorts.
Table 2
Univariable and multivariable analysis for comorbidities associated with overall survival in the development cohort.
Table 3
The final scoring model in the development cohort.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download