This article has been
cited by other articles in ScienceCentral.
Abstract
Background
This study evaluated the outcomes of patients with refractory/relapsed Hodgkin lymphoma (RRHL) treated with a bendamustine-based regimen in combination with ifosfamide, etoposide, and vinorelbine (VIBE).
Methods
Consecutive RRHL patients who were treated with the VIBE regimen were identified and studied for clinicopathologic characteristics, response to VIBE regimen, event-free survival (EFS), and feasibility of an autologous stem-cell transplant (autoSCT).
Results
In total, 24 patients received the VIBE regimen, and a median of 3 cycles were administered. In this cohort, 80% of the patients had received ≥2 prior lines of therapy. The overall and complete response rates with VIBE were 79% and 42%, respectively. After a median follow-up (following VIBE regimen) of 14 months (range, 3‒76), the 3-year EFS and OS were 46% and 74%, respectively. Of the eligible patients, 92% underwent successful AutoSCT. The mean CD34+ cell count in the autograft was 5.5×106/kg (SD 2.07). Neutropenia was the commonest hematologic toxicity and it was observed in 42% of the patients. However, only 9% of the patients developed grade III/IV febrile neutropenia. Chemotherapy-induced nausea and vomiting were the second most common grade III/IV toxicities in our cohort of patients.
Conclusion
In this retrospective analysis, the combination regimen, VIBE, has shown good efficacy in heavily pre-treated patients with RRHL without compromising stem cell collection. These encouraging results provide a rationale for further development of this regimen.
Keywords: Relapsed refractory, Hodgkin Lymphoma, Salvage, Bendamustine, Autologous transplant
INTRODUCTION
Classical Hodgkin lymphoma (cHL) is a highly chemo-responsive malignancy. As a result, high cure rates of approximately 90% for during the early stages and 65–70% for during the advanced stages of cHL have been reported [
1,
2]. Despite the excellent prognosis, a low yet significant number of patients do not respond to first-line chemotherapy or experience relapse after remission. The treatment of refractory or relapsed Hodgkin lymphoma (RRHL) is challenging, and the current standard of care is autologous stem cell transplantation (AutoSCT) if a salvage regimen produces a complete or near-complete response before transplantation.
The majority of second-line regimens used for HL are derived from regimens used for non-Hodgkin lymphoma. They can be broadly classified as platinum-based like ICE (ifosfamide, carboplatin, etoposide) [
3], DHAP (dexamethasone, cytarabine, cisplatin) [
4], or gemcitabine based like GVD (gemcitabine, vinorelbine, and pegylated liposomal doxorubicin) [
5] and GDP (gemcitabine, dexamethasone, cisplatin) [
6]. The complete response rates reported for these salvage regimens in patients with RRHL are less than 40% [
7]. This implies that even with these multiagent salvage chemotherapy regimens for RRHL, several patients do not respond optimally, and there is significant room for further improvement in this area. The patients who fail a second-line regimens have even more dismal outcomes. Brentuximab vedotin (BV)-based treatment regimens are emerging as an effective choice for RRHL. In a pivotal trial, the response rate with the use of single-agent brentuximab in patients with RRHL was reported to be 75% [
8]. More recently, the combination of BV with the immune checkpoint inhibitor nivolumab has shown promising results, with response rates reaching up to 82% [
9]. However, in a low-mid-income country (LMIC), these novel agents may not be available or their high cost precludes them from being used as part of a salvage regimen for a large majority of the patients even if they are available. Therefore, in LMICs, efficacious, as well as affordable, salvage regimens are always needed for this subset of patients.
Bendamustine comprises a 2-chloroethylamine alkylating moiety and a benzimidazole ring that imparts antimetabolite effects such as cladribine [
10]. The structural similarities of bendamustine with two different classes of anticancer agents make it a suitable candidate for synergistic combinations with other classes of anticancer drugs. Bendamustine has shown remarkable efficacy in patients with B-cell NHL. There are a few phase II data on the efficacy of single-agent bendamustine 120 mg/m
2 in patients with RRHL with an impressive complete response rate of 33% [
10].
We hypothesized that a bendamustine-based multiagent combination regimen would be effective in patients with RRHL. In this study, we present our experience with a bendamustine-based combination labeled VIBE (V=vinorelbine, I=ifosfamide, B=bendamustine, E=etoposide).
MATERIALS AND METHODS
In light of the emerging data on the efficacy of bendamustine, a group of experts at our center decided to treat RRHL patients with a combination of non-cross-resistant and not previously used conventional chemotherapeutic drugs, including vinorelbine, ifosfamide, bendamustine, and etoposide (VIBE), after obtaining informed consent.
To select the cases for this analysis, we retrospectively identified patients with RRHL who had received the VIBE regimen between January 2014 and December 2020. The chemotherapy drugs and their doses were as follows: bendamustine 90 mg/m2, Day 1–2; vinorelbine 30 mg/m2 IV Day 1 and 8; ifosfamide in 1,000 mg/m2 IV Day 1 to 3 (along with mesna), etoposide 60 mg/m2 on days 1 to 3, and pegylated subcutaneous G-CSF 6 mg on day 4 of therapy. The subsequent courses were repeated every 3 weeks or at the time of count recovery. The day 1–3 infusions were administered following admission to the inpatient facility, and the day 8 vinorelbine was administered in the outpatient clinic. The patients were followed up in our outpatient clinic to assess toxicity and response to therapy. The response assessment was performed after 2–4 courses as per the discretion of the treating physician. As this is a retrospective analysis of data, the response assessment could not be performed at a fixed time point.
For the subset of patients who underwent autoSCT after VIBE therapy, we collected additional data for stem cell mobilization, CD34+ cell yield, and engraftment status. We aimed to study the impact of a bendamustine-based salvage regimen on stem cell mobilization in this heavily pre-treated group of patients.
RESULTS
Patient characteristics
From our lymphoma database, we identified 24 patients who had received a bendamustine-based VIBE salvage regimen. The median age of the patients was 25 years (range, 13–60 yr). The male-to-female ratio was 3:1. All patients had an Eastern Cooperative Oncology Group performance status (PS) score of 0 or 1. Regarding histologic subtypes, 13 (54%) patients had the nodular sclerosis variant of cHL, 4 (16.6%) patients had cHL-mixed cellularity, and the remaining 8 patients had HL-unclassified. The median number of prior lines of anti-lymphoma therapies was 2 (range, 1–4), and 80% of the patients had received two or more prior lines of therapy. The baseline data for the patient-related and disease-related characteristics are summarized in
Table 1.
Treatment and outcome
In total, 68 courses of the VIBE regimen were administered to the patients. The median number of courses of the VIBE regimen administered per patient was 3 (range, 2–4). The overall clinical response was observed in 19 (79%) patients; 42% achieved a complete response (CR), 33% achieved a complete response-unconfirmed (CRu), and 4% achieved a partial response (PR). Five patients (20%) had stable/progressive disease at the completion of the VIBE regimen. Of the 19 patients with a favorable response, 12 (63.2%) received autoSCT successfully.
Univariate analysis showed that younger patients (age cut-off value <25 yr) had a higher CR/CRu rate (91% vs. 61.5%,
P=0.09). Patients with relapsed cHL had higher CR/CRu rates (85.7%) than patients with primary refractory cHL (70.5%,
P=0.4). The patients who had received <2 prior lines of therapy had a CR/CRu rate of 83%, while the patients with ≥2 lines failure showed CR/CRu rates of 73% (
P=0.1). Similarly, patients with stage IV disease at the time of starting the VIBE regimen had poorer CR/CRu rates at 62% in comparison to patients with stage II or III disease (83% and 80%, respectively;
P=-0.4) (
Fig. 1).
Stem cell mobilisation
Among the 19 patients with CR/CRu, 12 (63.2%) underwent an autologous stem cell transplantation. In these patients, the stem cells were mobilized with GCSF with or without plerixafor, which resulted in successful mobilization in 11 (92%) patients.
The median age of the patients who underwent autoSCT was 21 years (range, 13–29), and the median number of stem-cell harvests required was 2 (range, 1–4). Eight patients needed plerixafor assisted mobilization, and the median number of plerixafor injections used was 1 (range, 1–3).
Peripheral blood CD34+ cell count on day 4 of G-CSF mobilization was available for eight patients, and the median day-4 CD34+ count was 8.18/mL (range, 1–22). The mean of the total number of CD34+ cells collected was 5.17×106/kg (SD, 2.07). The mean of CD34+ cells collection at the time of first apharesis was 3.36×106/kg (SD, 2.21). All but one patient had successful mobilization during the first attempt with GCSF+/-plerixafor. The patient who failed GCSF+plerixafor mobilization underwent successful AutoSCT after 1 month using a chemo-mobilization strategy.
The median number of days of neutrophil engraftment was 11 (range, 9–13). One patient had engraftment failure and died due to infection in the state of aplasia on day +90. The CD34+ count in the stem cell graft, in this case, was 2.5×106/kg. This patient was in a state of CR at the time of transplant and prior to transplant, he had received 4 lines of therapy.
Toxicity
The VIBE regimen was fairly well-tolerated, and neutropenia was the most common form of hematologic toxicity observed in 42% of patients. Only 18% of patients reported grade III/IV toxicity (neutropenia and vomiting) according to the Common Terminology Criteria for Adverse Events v4.0. There were no chemotoxicity-related deaths during therapy.
Ten patients (42%) developed febrile neutropenia, and only two of them developed grade IV febrile neutropenia requiring hospitalization and intravenous antibiotics. The remaining 8 patients developed low-risk neutropenia (ANC>1,000/mcl and no organ dysfunction), and they improved with oral antibiotics administered on an outpatient basis. Chemotherapy-induced nausea and vomiting (CINV) was the most common form of non-hematologic toxicity observed in 14 patients (58%). Two of these patients developed grade III CINV requiring treatment with intravenous fluid and extended intravenous 5-HT3 antagonists. Diarrhea and oral mucositis were observed in 17% and 29% of patients, respectively. None of the patients required hospitalization for diarrhea or mucositis.
Survival
The median follow-up after starting the VIBE regimen was 14 months (range, 3–76 mo). The 3-year EFS and OS rates were 46% and 74%, respectively. The median event-free survival of the whole group was 28 months, while the median was not reached for OS. There was a survival advantage for patients who achieved CR following the VIBE regimen. The median EFS for patients attaining a complete response was 37 months; in comparison, the EFS for patients who could not achieve a CR was 6 months (
P=0.0003) (
Fig. 2B).
Similarly, younger patients (<25 yr) had a 3-year EFS value of 90%; in comparison, older patients (≥25 yr) had a 3-year EFS value of only 22.4% (
P=0.04). The 3-year EFS for the patients undergoing autoSCT was 51.4%, and that for those who did not undergo autoSCT was 46% (
P=0.2) (
Fig. 2C, D).
DISCUSSION
The outcome of patients with cHL who have a primary refractory or relapsed disease remains suboptimal. The response rates of bendamustine- and vinorelbine-based salvage regimens for RRHL have been assessed in only a few studies [
10-
12].
In the current study, the VIBE regimen was administered as a third-line regimen in 80% of the patients, and 70% of the patients had primary refractory HL. The details about prior treatment regimen received by each patients are presented in
Table 2. Understandably, this subset of patients is likely to have much poorer outcomes than patients who have failed only frontline treatment with ABVD. The results observed with the VIBE regimen in this high-risk group were encouraging. Approximately 42% of patients achieved metabolic CR documented by a PET-CT scan at the end of therapy, and 33% had a near-complete response. These results compare favorably with other chemotherapy-based salvage regimens such as ICE/DHAP/ GDP/GVD, which have shown CR rates within the range of 20–25% [
4,
7]. We compared our results in detail with two relatively newer salvage chemotherapy regimens known as IGEV (ifosfamide, gemcitabine, etoposide, and vinorelbine) and BeGEV (bendamustine, gemcitabine, and vinorelbine), which have reported CR rates of 54% and 73%, respectively [
11,
12].
The IGEV regimen was first studied by an Italian group, and it produced a CR rate of 53% and an overall response rate of 81% in a study of 49 patients with RRHL. After IGEV, stem cell collection with a strategy of chemo-mobilization for all patients was excellent, with a 98% success rate [
11]. Overall, the IGEV-related toxic effects were mild, with a relatively low incidence of grade 3 and 4 toxicities according to the WHO Common Toxicity Criteria. In this study, 28% of the patients developed grade 3/4 neutropenia, and only one patient required hospital admission for management. This pattern is quite similar to our observations of good tolerability of the VIBE regimen and negligible admission rate for the management of chemotoxicity.
To further improve results from IGEV, in a relatively recent report, Santoro
et al. [
12] replaced ifosfamide with bendamustine and developed a regimen called a BeGEV regimen. In a study of 59 patients who received the BeGEV regimen, the complete remission rate was 73%, the overall response rate was 83%; the 2-year-PFS was 62.2%. Eighty-seven percent of the responding patients underwent successful autoSCT [
12]. The BeGEV regimen was also well-tolerated, and the most common non-hematologic toxicity was grade 1 to 2 nausea and vomiting. Grade 3 or 4 febrile neutropenia was observed in only 14% of patients in this study. The incidence of grade 3 or 4 febrile neutropenia with the VIBE regimen in the current study was 8.3%.
With the VIBE regimen, we observed a CR rate of 42% and an ORR of 79%. The estimated two-year event-free survival rate was 61%. Only 68% of the responding patients underwent successful auto-HSCT in our study cohort. The reasons for the seemingly low (68%) rate of autologous transplant in our study cohort was low affordability or lack of social support in 21% patients (4/19 of the responding patients). Sixteen percent patients (3/19 cases) refused to undergo auto-HSCT due to their personal preference based on the risk associated with the procedure. It has been reported that bendamustine interferes with stem cell mobilization, which may impact the transplant outcome in patients with various lymphomas [
13,
14]. However, our experience with VIBE was contrary to this notion; in 92% of patients, adequate stem cells were collected and engraftment was achieved. There was only one case of primary engraftment failure.
The VIBE regimen was well-tolerated, and no chemotoxicity-related death was observed. As expected from the constituent drugs, febrile neutropenia and chemotherapy-induced nausea and vomiting were the two most common toxicities. The pattern and severity of toxicity observed by the VIBE regimen were similar to those observed with the IGEV and BeGEV regimens.
It is remarkable to mention that in both the studies published with the IGEV and BeGEV regimens, the patients had received only one prior line of therapy. In the current study, the median number of prior regimens was 3, and 70% of the patients had received ≥2 lines of treatment before the treatment with the VIBE regimen. Although cross-trial comparisons cannot be used to draw definite conclusions, the achievement of an overall response rate of 79.2% in a subset of patients who have failed regimens such as GDP/DHAP in the past points towards a higher synergism among the constituent drugs in the VIBE regimen and makes an interesting case for further evaluation of the same.
Table 3 [
6,
8,
9,
11,
12,
15] presents a comparative illustration of some key salvage regimens along with their response rates, stem cell mobilization success rates, and survival rates.
Within the last few years, there have been remarkable reports on the treatment of RRHL using modern targeted drugs, such as antiCD30 monoclonal antibody-drug conjugate brentuximab vedotin (BV) as a single agent [
16] or in combination with an immune checkpoint inhibitor, nivolumab, and pembrolizumab [
17]. In the phase II study, a CR rate of 34% and an ORR of 75% were observed with single-agent BV in patients with RRHL who had received a median of 3.5 prior lines of therapy. These results are remarkable for a single agent and chemotherapy-free regimen. It is exciting to have a better regimen to treat this very difficult subset of cHL. However, due to the higher cost and unavailability of these drugs in most LMICs, these newer options remain inaccessible to a large number of patients. Therefore, the scope for redesigning a combination of conventional anticancer drugs to improve efficacy remains. Our observations with the VIBE regimen indicate better efficacy, even in patients with disease refractory to multiple lines of therapy. Our results with the VIBE regimen need to be validated at other centers and with a larger cohort of patients.
It is likely that the addition of these newer biologic agents to the current VIBE chemotherapy regimen may further improve the response rates in patients with RRHL. However, the same needs to be prospectively studied.
The limitations fundamental to retrospective data analysis are also applicable to this study. The study had a relatively small sample size of 24 patients since it only involved a single center, and RRHL is uncommon. The other limitation was that the post VIBE therapy assessment could not be conducted after a fixed number of courses because this was a retrospective analysis.
CONCLUSIONS
The ideal salvage regimen for relapsed/refractory Hodgkin lymphoma remains elusive. Currently, various regimens such as ICE, DHAP, GDP, and BeGV are preferred. However, most of the published data on these regimens are from patients treated after the failure of first-line therapy. The VIBE regimen, analyzed in this study, has shown remarkable efficacy and tolerability in a heavily pre-treated cohort of patients with RRHL (80% of these patients had failed ≥2 lines of therapy). This combination did not adversely affect stem cell yield in patients undergoing autologous stem cell transplantation. In a large part of the world, there is limited access to targeted anti-CD30 mAb and immunotherapy. Therefore, the outcomes with the novel combination of conventional chemotherapy drugs, VIBE, are promising for this difficult-to-treat subset of patients. These results provide a rationale for further development of this regimen and larger prospective multi-center studies.
ACKNOWLEDGMENTS
The authors acknowledge the data entry operator and medical record department of our institute for helping with data collection and analysis.
REFERENCES
1. Canellos GP, Rosenberg SA, Friedberg JW, Lister TA, Devita VT. 2014; Treatment of Hodgkin lymphoma: a 50-year perspective. J Clin Oncol. 32:163–8. DOI:
10.1200/JCO.2013.53.1194. PMID:
24441526.

2. Straus DJ, Portlock CS, Qin J, et al. 2004; Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood. 104:3483–9. DOI:
10.1182/blood-2004-04-1311. PMID:
15315964.

3. Hertzberg MS, Crombie C, Benson W, Taper J, Gottlieb D, Bradstock KF. 2003; Outpatient-based ifosfamide, carboplatin and etoposide (ICE) chemotherapy in transplant-eligible patients with non-Hodgkin's lymphoma and Hodgkin's disease. Ann Oncol. 14(Suppl 1):i11–6. DOI:
10.1093/annonc/mdg703. PMID:
12736225.

4. Abali H, Urün Y, Oksüzoğlu B, et al. 2008; Comparison of ICE (ifosfamide-carboplatin-etoposide) versus DHAP (cytosine arabinoside-cisplatin-dexamethasone) as salvage chemotherapy in patients with relapsed or refractory lymphoma. Cancer Invest. 26:401–6. DOI:
10.1080/07357900701788098. PMID:
18443961.
5. Bartlett NL, Niedzwiecki D, Johnson JL, et al. 2007; Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 18:1071–9. DOI:
10.1093/annonc/mdm090. PMID:
17426059.

6. Kuruvilla J, Nagy T, Pintilie M, Tsang R, Keating A, Crump M. 2006; Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer. 106:353–60. DOI:
10.1002/cncr.21587. PMID:
16329112.

8. Younes A, Gopal AK, Smith SE, et al. 2012; Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 30:2183–9. DOI:
10.1200/JCO.2011.38.0410. PMID:
22454421. PMCID:
PMC3646316.
9. Herrera AF, Moskowitz AJ, Bartlett NL, et al. 2018; Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 131:1183–94. DOI:
10.1182/blood-2017-10-811224. PMID:
29229594. PMCID:
PMC5855021.

10. Moskowitz AJ, Hamlin PA Jr, Perales MA, et al. 2013; Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 31:456–60. DOI:
10.1200/JCO.2012.45.3308. PMID:
23248254. PMCID:
PMC3862960.

11. Santoro A, Magagnoli M, Spina M, et al. 2007; Ifosfamide, gemcitabine, and vinorelbine: a new induction regimen for refractory and relapsed Hodgkin's lymphoma. Haematologica. 92:35–41. DOI:
10.3324/haematol.10661. PMID:
17229633.

12. Santoro A, Mazza R, Pulsoni A, et al. 2016; Bendamustine in combination with gemcitabine and vinorelbine is an effective regimen as induction chemotherapy before autologous stem-cell transplantation for relapsed or refractory Hodgkin lymphoma: final results of a multicenter phase II study. J Clin Oncol. 34:3293–9. DOI:
10.1200/JCO.2016.66.4466. PMID:
27382096.

13. Gac AC, Azar N, Daguindau E, et al. 2016; Does bendamustine impact the mobilization of peripheral blood stem cells? A multicenter retrospective study of 23 cases. Leuk Lymphoma. 57:1149–53. DOI:
10.3109/10428194.2016.1140160. PMID:
26879408.

14. Villa D, Sehn LH, Savage KJ, et al. 2020; Bendamustine and rituximab as induction therapy in both transplant-eligible and -ineligible patients with mantle cell lymphoma. Blood Adv. 4:3486–94. DOI:
10.1182/bloodadvances.2020002068. PMID:
32735654. PMCID:
PMC7422137.

15. Moskowitz CH, Nimer SD, Zelenetz AD, et al. 2001; A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 97:616–23. DOI:
10.1182/blood.V97.3.616. PMID:
11157476.

16. O'Connor OA, Lue JK, Sawas A, et al. 2018; Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin's lymphoma: an international, multicentre, single-arm, phase 1-2 trial. Lancet Oncol. 19:257–66. DOI:
10.1016/S1470-2045(17)30912-9. PMID:
29276022.
17. Armand P, Engert A, Younes A, et al. 2018; Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II checkmate 205 trial. J Clin Oncol. 36:1428–39. DOI:
10.1200/JCO.2017.76.0793. PMID:
29584546. PMCID:
PMC6075855.

Fig. 1
Y-axis: CR/CRu rates (expressed in percentage); X-axis: various variables.
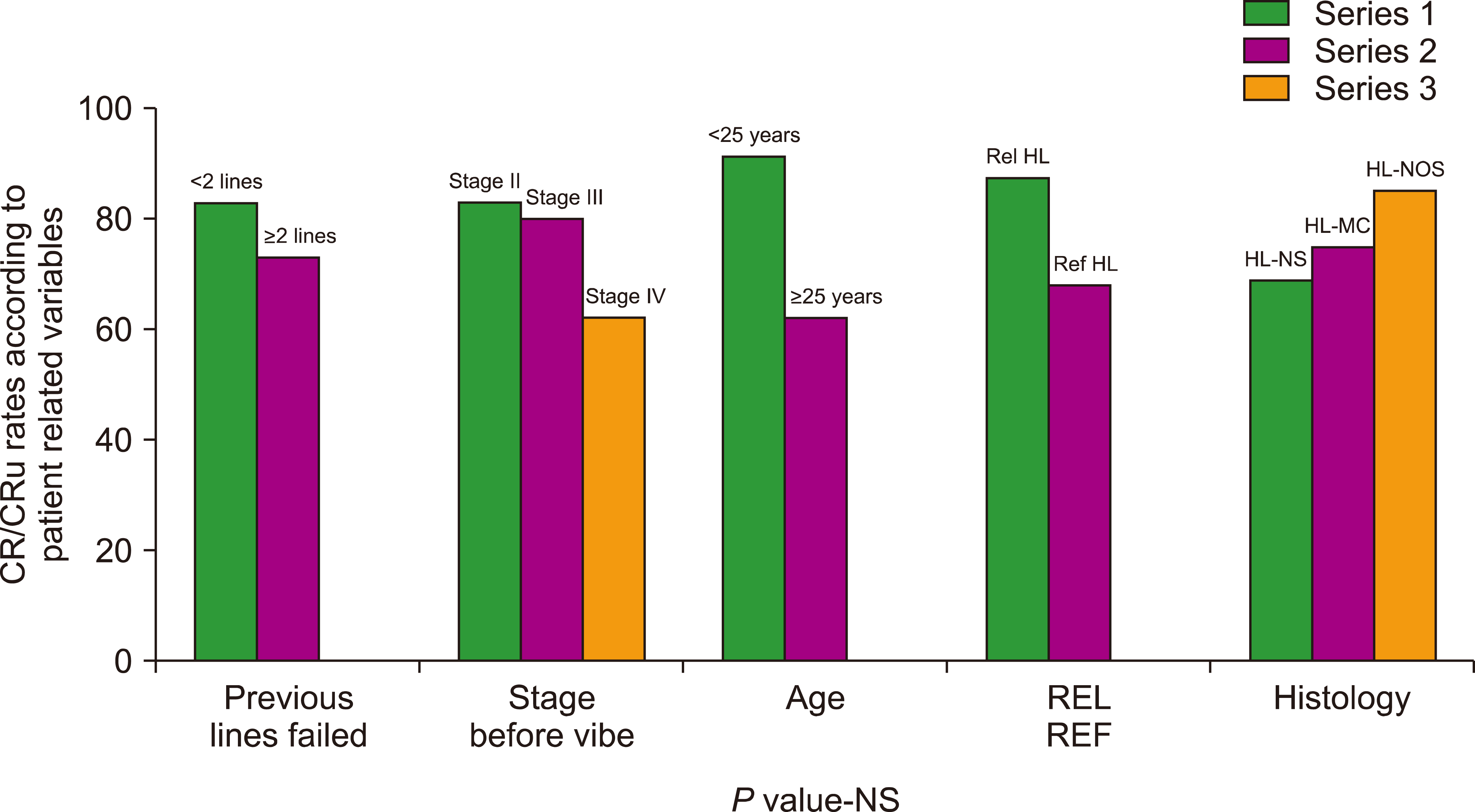
Fig. 2
Kaplan-Meier survival curve for event-free survival (A). Whole group. EFS according to response to VIBE therapy (B). EFS according to age group (C). EFS according to AutoHSCT status (D).
Table 1
Patient characteristics and disease-related variables.
|
Parameters |
N (%) |
|
Age, years, median (range) |
25 (13–60) |
|
Gender ratio |
M:F=3:1 |
|
B-symptoms |
13 (54) |
|
Bulky disease |
5 (20.8) |
|
Extranodal disease |
5 (20.8) |
|
cHL subtypes |
|
|
Nodular sclerosis |
13 (54) |
|
Mixed cellularity |
4 (16.6) |
|
Unclassified |
7 (33.3) |
|
Previous 2 lines failed |
19 (80) |
|
Stage at the time of starting VIBE stage |
|
|
Stage II |
6 (25) |
|
Stage III |
10 (41.6) |
|
Stage IV |
8 (33.3) |
|
Numbers of VIBE regimen received |
|
|
Two courses |
9 (37.5) |
|
Three courses |
6 (25) |
|
Four courses |
9 (37.5) |
Table 2
Characteristics of individual patients.
|
No. |
Age/
sex |
Stage at the time of diagnosis |
Prior regimen used |
Previous N of therapy |
Diagnosis to VIBE (mo) |
Stage before VIBE |
VIBE cycles |
Response after VIBE |
Auto HSCT
yes/no |
|
Relapsed cases of cHL |
|
1 |
21/F |
II-A |
ABVD×6 (2012)-CR, IFRT 30Gy, GDP×4 (2013)-PD |
3 |
22 |
III |
2 |
CR |
Yes |
|
2 |
28/M |
II-B |
ABVD×6 (2014)-CR, GDP×3 (2016)-PR |
2 |
31 |
III |
2 |
CRU |
Yes |
|
3 |
13/M |
II-A |
ABVD×6 (2013)-CR, GDP×4 (2016)-SD |
2 |
54 |
III |
2 |
CRU |
Yes |
|
4 |
25/M |
II-AX |
ABVD×6 (2014)-CR, Bendamustine×2, RT (PR)
GDP×2-PR, Nivolumab×8 doses-PR but soon progressed |
2 |
41 |
III |
2 |
PD |
No |
|
5 |
15/M |
II-B |
ABVD×6 (2010)-CR, IEV×4 RT to mediastinum (2012)-CR, GDP×2 (2019)-SD |
4 |
90 |
III |
3
|
CR |
Yes |
|
6 |
13/M |
III-B |
ABVD×6 (2016)-CR
GDP×2 (2019)-PD |
2 |
44 |
III |
3 |
CRU |
No |
|
7 |
29/M |
III-BS |
ABVD×6 (2016)-CR |
1 |
48 |
III |
4 |
CRU |
Yes |
|
Primary refractory cases of cHL |
|
8 |
21/M |
III-BEX |
ABVD×4 (2013)-PR, BEACOPP×3 (2013)-PR, IFRT 30 Gy, GDP× (2014)-PR |
4 |
20 |
I |
2 |
CR |
Yes |
|
9 |
20/M |
III-B |
COPP×1
BEACOPP×5,-PD |
2 |
6 |
III |
2 |
PD |
No |
|
10 |
60/M |
IV -BS |
ABVD×6 (2016)-PD, Splenectomy |
2 |
16 |
IV |
4 |
CR |
No |
|
11 |
27/M |
IV-AE |
CHV+Bleomycin, Etoposide×6 (2014)-PR
DHAP×2 (2014)-PD, ICE×4 (2015)-SD |
3 |
27 |
IV |
4 |
CR |
Yes |
|
12 |
26/M |
IV-BE |
ABVD×6 (2016)-PD |
1 |
8 |
IV |
3 |
CRU |
Yes |
|
13 |
33/M |
IV AEX |
ABVD×4 (2015)-SD,
GDP×4 (2016)-PD |
2 |
20 |
IV |
4 |
CRU |
Yes |
|
14 |
27/M |
II-A |
ABVD×4 (2017)-PD |
1 |
5 |
III |
4 |
CR |
Yes |
|
15 |
18/M |
III BX |
ABVD×4 (2015)-SD |
1 |
5 |
III |
3 |
CR |
No |
|
16 |
27/F |
II-BX |
ABVD×6 (2017) PR, GDP×2 (2018)-PR, IFRT-PD |
3 |
13 |
I |
4 |
PR |
No |
|
17 |
21/F |
IV-B |
ABVD×6 (2018) PR, GDP×2 (2019)-PR |
2 |
12 |
IV |
3 |
CRU |
No |
|
18 |
21/M |
III-B |
ABVD×6 (2018) PD, GDP×4 (2019)-PD |
2 |
10 |
III |
3 |
CR |
Yes |
|
19 |
26/M |
IVB |
ABVD×5 (2018)-SD
GDP×2 (2019)-PR |
2 |
24 |
IV |
4 |
PD |
No |
|
20 |
25/M |
IVBE |
COPP×1
ABVD×5 (2019)-PD
GDP×2 (2019)-PD |
2 |
15 |
IV |
2 |
SD |
No |
|
21 |
14/F |
IVA |
COPP×2 (2019)-PR
ABVD×6 (2020)-PD |
2 |
13 |
II |
3 |
CR |
Yes |
|
22 |
28/F |
IVBS |
ABVD×6 (2019)-PR |
1 |
25 |
IV |
4 |
PD |
No |
|
23 |
22/f |
II-B |
ABVD×6 (2018)-PD, GDP×2 (2019)-SD |
2 |
19 |
IV |
2 |
CRU |
No |
|
24 |
25/m |
III-A |
ABVD×6 (2012-13)-PD
COPP×6 (2013)-PD
IFRT 10 Fr (2015), CEP×3 (2016)-PR |
4 |
60 |
III |
2 |
CR |
No |
Table 3
Comparison of various salvage regimens for relapsed or refractory Hodgkin lymphoma.
|
Regimen |
Median lines failed |
CR (%) |
ORR (%) |
Successful stem cell mobilisation (%) |
PFS |
|
ICE [15] |
1 |
26 |
88 |
96 |
58% at 3.5 yr |
|
GDP [6] |
1 |
10 |
62 |
97 |
74% at 18 mo |
|
miniBEAM [6] |
1 |
20 |
68 |
57 |
35% at 18 mo |
|
IGEV [11] |
1 |
54 |
81 |
98 |
NR |
|
BeGEV [12] |
1 |
73 |
83 |
96 |
62% 2 yr |
|
BV [8] |
3.5 |
34 |
75 |
NR |
22% at 5 yr |
|
BV+nivolumab [9] |
1 |
62 |
85 |
NR |
NR |
|
VIBE current study
|
3 |
42 |
79 |
92 |
61% 2 yr, EFS |





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download