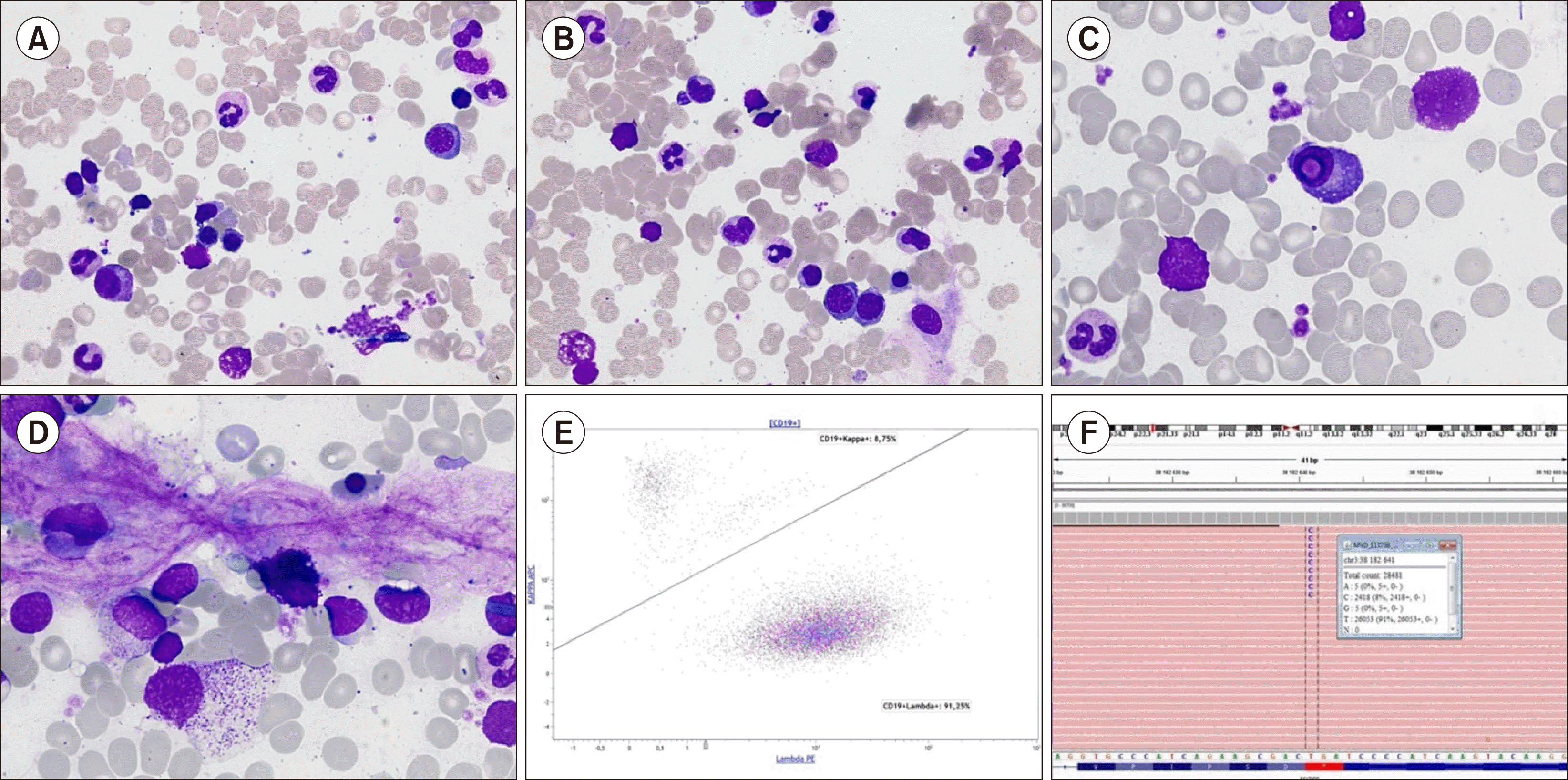
A 79-year-old male was referred for non-regenerative anaemia (haemoglobin, 93 g/L). His blood examination revealed normal platelet (373×109/L) and WBC (6.4×109/L) count and differentials (neutrophils, 64%; lymphocytes, 21%; monocytes, 15%). Bone marrow examination did not reveal dysplastic changes or excess blasts (A and B, May-Grünwald-Giemsa, ×630). Lymphocytes represented 9% of total cells with normal morphology. The proportion of plasma cells was not increased (2%) but some possessed intranuclear pseudo-inclusions, known as Dutcher bodies (C, ×1,000), without any morphological changes commonly related to malignancy (such as nuclear-cytoplasmic asynchrony). Some mast cells were present (D, ×1,000). Despite the absence of atypical lymphoid involvement, these findings suggested the presence of mature B cell neoplasm, especially lymphoplasmacytic lymphoma (LPL). Flow cytometric immunophenotyping revealed lambda monotypic B cells population (E) representing 1% of leukocytes with co-expression of CD19, CD20, and CD79a, without CD5, CD23, and CD10. Molecular genetic analysis revealed MYD88 L265P mutation with 8% VAF (F). These results were indicative of LPL. Serum protein electrophoresis revealed IgM lambda monoclonal spike (8 g/L) further indicating towards Waldenström’s macroglobulinaemia. LPL diagnosis can be difficult, especially in the absence of lymphocytosis, bone marrow involvement, and evocative clinical features. The coexistence of plasma cells with Dutcher bodies and mast cells in bone marrow should thus be provocative to diagnose LPL.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download