INTRODUCTION
METHODS
Materials
Cell culture
Cell viability
Measurement of apoptosis
Collagen content
ALP activity
Mineralization
Measurements of inositol-requiring 1 (IRE1) and activating transcription factor 6 (ATF-6) levels
Measurements of tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 levels
Measurements of glyoxalase I activity and reductions in glutathione (GSH) levels
Measurement of intracellular reactive oxygen species (ROS) levels
Measurement of mitochondrial superoxide levels
Measurement of cardiolipin peroxidation
Determination of the mitochondrial membrane potential (MMP)
Measurement of adenosine triphosphate (ATP) levels
Measurement of proliferator-activated receptor gamma coactivator 1α (PGC-1α) levels
Measurement of nitric oxide (NO) generation
Statistical analysis
RESULTS
Cytoprotective effects of spironolactone on MG-induced cytotoxicity in osteoblastic MC3T3-E1 cells
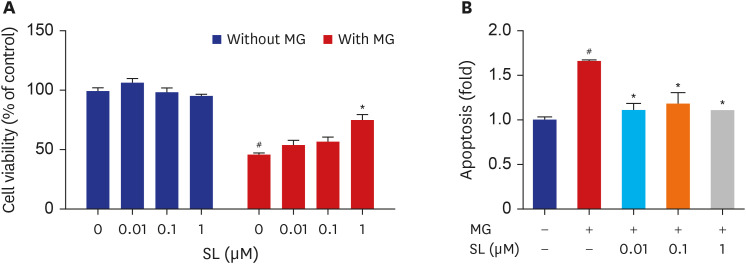 | Fig. 1Effects of spironolactone on the MG-induced cytotoxicity in MC3T3-E1 cells. Osteoblasts were treated with SL in the absence or presence of 400 μM of MG for 48 hours, and then (A) cell viability and (B) apoptosis were analyzed. Data are expressed as the mean ± standard error of mean (n = 6).MG = methylglyoxal, SL = spironolactone.
#P < 0.05 compared to untreated cells; *P < 0.05 compared to cells treated with MG alone.
|
Effects of spironolactone on osteoblast differentiation in MG-treated MC3T3-E1 cells
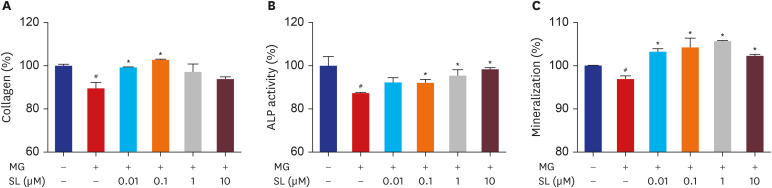 | Fig. 2Effects of SL on MG-induced osteoblast dysfunction in osteoblastic MC3T3-E1 cells. Cells were treated 10 mM of β-glycerophosphate and 50 μg/mL of ascorbic acid to initiate differentiation. After six days, osteoblasts were treated with SL in the absence or presence of 400 μM of MG for 48 hours, and then (A) collagen content and (B) ALP activity were measured. (C) After 14 days, osteoblasts were treated with SL in the absence or presence of 400 μM of MG for 48 hours, and then mineralization levels were measured. The control values for collagen content, ALP activity, and mineralization were 46.27 ± 0.346 μg/106 cells, 0.502 ± 0.022 unit/mg protein, and 0.654 ± 0.0004 OD/106 cells, respectively. Data are expressed as the mean ± standard error of mean (n = 6).MG = methylglyoxal, SL = spironolactone, ALP = alkaline phosphatase.
#P < 0.05 compared to untreated cells; *P < 0.05 compared to cells treated with MG alone.
|
Effects of spironolactone on ER stress and the generation of inflammatory cytokines in MG-treated MC3T3-E1 cells
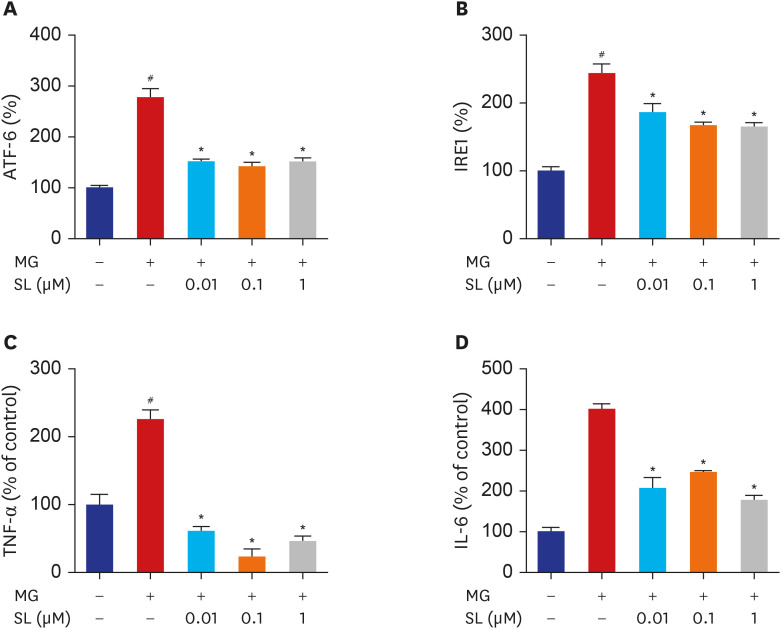 | Fig. 3Effects of SL on ER stress, TNF-α and IL-6 production in MG-treated MC3T3-E1 cells. Osteoblasts were pre-incubated with SL before treatment with 400 μM of MG for 24 hours. The control values for ATF6 and IRE1 were 54.72 ± 0.498 and 3.095 ± 0.179 ng/mg, respectively. The control values for TNF-α and IL-6 were 18.09 ± 2.713 pg/mg and 0.655 ± 0.033 ng/mg, respectively. Data are expressed as the mean ± standard error of mean (n = 6).ATF = activating transcription factor 6, MG = methylglyoxal, SL = spironolactone, IRE1 = inositol-requiring 1, TNF-α = tumor necrosis factor-α, IL = interleukin.
#P < 0.05 compared to untreated cells; *P < 0.05 compared to cells treated with MG alone.
|
Effects of spironolactone on glyoxalase I activity and GSH levels in MG-treated MC3T3-E1 cells
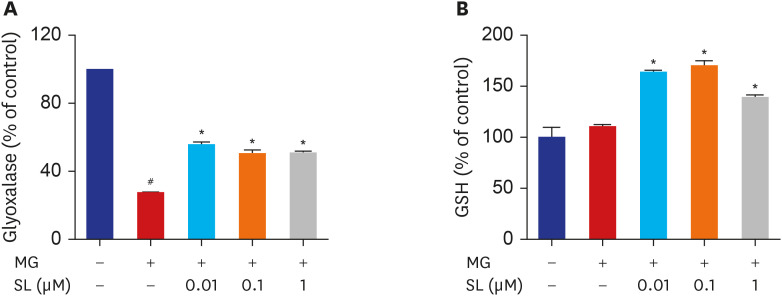 | Fig. 4Effects of SL on glyoxalase I activity and reduced GSH levels in MG-treated cells. Osteoblasts were pre-incubated with SL before treatment with 400 μM of MG for 48 hours. The control values for (A) glyoxalase I activity and (B) reduced GSH levels were 0.29 ± 0.001 ΔOD/min/mg and 72.03 ± 6.91 μg/mg, respectively. Data are expressed as the mean ± standard error of mean (n = 6).MG = methylglyoxal, SL = spironolactone, GSH = glutathione.
#P < 0.05 compared to untreated cells; *P < 0.05 compared to cells treated with MG alone.
|
Effects of spironolactone on oxidative stress in MG-treated MC3T3-E1 cells
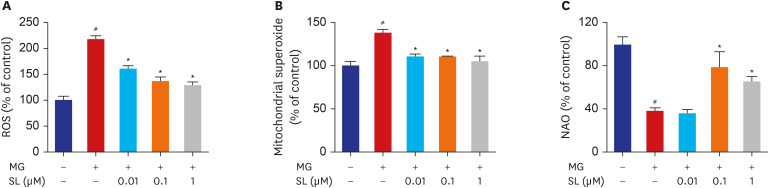 | Fig. 5Inhibitory effects of SL on MG-induced oxidative stress in MC3T3-E1 cells. Osteoblasts were pre-incubated with SL before treatment with 400 μM of MG for 48 hours. (A) Changes in ROS levels measured using the dichlorodihydrofluorescein fluorescence method. (B) Mitochondrial superoxide levels detected using the MitoSOX™ red mitochondrial superoxide indicator. (C) Cardiolipin oxidation measured using 5 µM NAO. All data are expressed as a percentage of the fluorescence emitted by bound NAO relative to untreated control cells. Note that a decrease in NAO binding is related to cardiolipin peroxidation. Data are expressed as the mean ± standard error of mean (n = 6).ROS = reactive oxygen species, MG = methylglyoxal, SL = spironolactone, NAO = 10-N-nonyl-acridin orange.
#P < 0.05 compared to untreated cells; *P < 0.05 compared to cells treated with MG alone.
|
Effects of spironolactone on mitochondrial function in MG-treated MC3T3-E1 cells
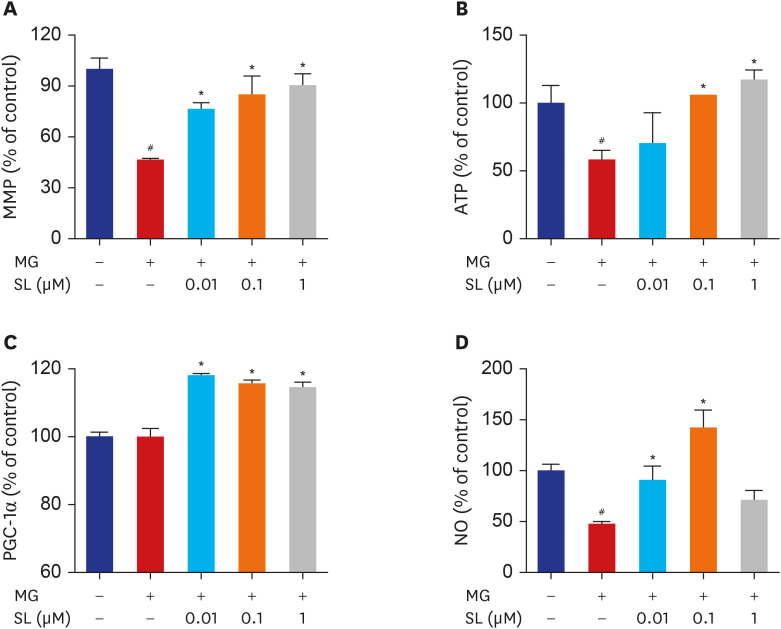 | Fig. 6Effects of SL on MG-induced mitochondrial dysfunction in osteoblastic MC3T3-E1 cells. Osteoblasts were pre-incubated with SL before treatment with 400 μM of MG for 48 hours. (A) Mitochondrial depolarization i.e., loss of MMP is represented by a decrease in the red/green fluorescence ratio; all data are expressed as a percentage of control values. The control values for (B) ATP levels and (C) PGC-1α levels were 1.496 ± 0.193 nmol/mg and 387.98 ± 5.39 ng/mg, respectively. All data for (D) NO are expressed as a mean relative percentage of fluorescence. Data are expressed as the mean ± standard error of mean (n = 6).MMP = mitochondrial membrane potential, MG = methylglyoxal, SL = spironolactone, ATP = adenosine triphosphate, PGC-1α = proliferator-activated receptor gamma coactivator 1α, NO = nitric oxide.
#P < 0.05 compared to untreated cells; *P < 0.05 compared to cells treated with MG alone.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download