1. Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008; 358(16):1700–1711. PMID:
18420502.

2. Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002; 288(6):728–737. PMID:
12169077.
3. Johnson S. Cognitive and behavioural outcomes following very preterm birth. Semin Fetal Neonatal Med. 2007; 12(5):363–373. PMID:
17625996.

4. Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009; 124(2):717–728. PMID:
19651588.

5. Taylor HG, Clark CA. Executive function in children born preterm: risk factors and implications for outcome. Semin Perinatol. 2016; 40(8):520–529. PMID:
27836424.

6. Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 2018; 172(4):361–367. PMID:
29459939.

7. Shim JW, Jin HS, Bae CW. Changes in survival rate for very-low-birth-weight infants in Korea: comparison with other countries. J Korean Med Sci. 2015; 30(Suppl 1):S25–S34. PMID:
26566354.

8. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92(4):529–534. PMID:
305471.

9. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986; 33(1):179–201. PMID:
3081865.

10. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163(7):1723–1729. PMID:
11401896.

11. Kwak KJ, Oh SW, Kim CT. Manual for Korean Wechsler Intelligence Scale for Children-IV (K-WISC-IV)-Manual. 4th ed. Seoul, Korea: Hakjisa;2011.
12. Shin MS, Cho S, Chun SY, Hong KE. A study of the development and standardization of ADHD diagnostic system. J Korean Acad Child Adolesc Psychiatry. 2000; 11(1):91–99.
13. Shin MS, Park MJ. Stroop Color and Word Test: a Manual for Clinical and Experimental Uses. Seoul, Korea: Hakjisa;2007.
14. Koo HJ, Shin MS. A standardization study of children's color trails test (CCTT). J Korean Acad Child Adolesc Psychiatry. 2008; 19(1):28–37.
15. Heaton RK. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL, USA: Psychological Assessment Resources;1993.
16. Nolan TM, Bond L, Adler R, Littlefield L, Birleson P, Marriage K, et al. Child behaviour checklist classification of behaviour disorder. J Paediatr Child Health. 1996; 32(5):405–411. PMID:
8933400.

17. So YK, Noh JS, Kim YS, Ko SG, Koh YJ. The reliability and validity of Korean parent and teacher ADHD rating scale. J Korean Neuropsychiatr Assoc. 2002; 41(2):283–289.
18. Youn Y, Lee SM, Hwang JH, Cho SJ, Kim EK, Kim EA, et al. National registry data from Korean neonatal network: two-year outcomes of Korean very low birth weight infants born in 2013–2014. J Korean Med Sci. 2018; 33(48):e309. PMID:
30473651.

19. Kim HS, Kim EK, Park HK, Ahn DH, Kim MJ, Lee HJ. Cognitive outcomes of children with very low birth weight at 3 to 5 years of age. J Korean Med Sci. 2020; 35(1):e4. PMID:
31898433.

20. Cheong JLY, Anderson PJ, Burnett AC, Roberts G, Davis N, Hickey L, et al. Changing neurodevelopment at 8 years in children born extremely preterm since the 1990s. Pediatrics. 2017; 139(6):e20164086. PMID:
28814550.

21. Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009; 123(6):1485–1492. PMID:
19482758.

22. Alloway TP, Alloway RG. Investigating the predictive roles of working memory and IQ in academic attainment. J Exp Child Psychol. 2010; 106(1):20–29. PMID:
20018296.

23. Burnett AC, Anderson PJ, Lee KJ, Roberts G, Doyle LW, Cheong JLY, et al. Trends in executive functioning in extremely preterm children across 3 birth eras. Pediatrics. 2018; 141(1):e20171958. PMID:
29196505.

24. Costa DS, Miranda DM, Burnett AC, Doyle LW, Cheong JLY, Anderson PJ, et al. Executive function and academic outcomes in children who were extremely preterm. Pediatrics. 2017; 140(3):e20170257. PMID:
28853418.

25. Beaino G, Khoshnood B, Kaminski M, Marret S, Pierrat V, Vieux R, et al. Predictors of the risk of cognitive deficiency in very preterm infants: the EPIPAGE prospective cohort. Acta Paediatr. 2011; 100(3):370–378. PMID:
21241364.

26. Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr. 2015; 169(12):1162–1172. PMID:
26457641.
27. Molloy CS, Anderson PJ, Anderson VA, Doyle LW. The long-term outcome of extremely preterm (<28 weeks' gestational age) infants with and without severe retinopathy of prematurity. J Neuropsychol. 2016; 10(2):276–294. PMID:
25809467.
28. Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet. 2013; 382(9902):1445–1457. PMID:
23782686.

29. Schmidt B, Davis PG, Asztalos EV, Solimano A, Roberts RS. Association between severe retinopathy of prematurity and nonvisual disabilities at age 5 years. JAMA. 2014; 311(5):523–525. PMID:
24496539.

30. Böhm B, Katz-Salamon M, Institute K, Smedler AC, Lagercrantz H, Forssberg H. Developmental risks and protective factors for influencing cognitive outcome at 5 1/2 years of age in very-low-birthweight children. Dev Med Child Neurol. 2002; 44(8):508–516. PMID:
12206615.

31. Glass TJA, Chau V, Gardiner J, Foong J, Vinall J, Zwicker JG, et al. Severe retinopathy of prematurity predicts delayed white matter maturation and poorer neurodevelopment. Arch Dis Child Fetal Neonatal Ed. 2017; 102(6):F532–F537. PMID:
28536205.

32. De Curtis M, Rigo J. Extrauterine growth restriction in very-low-birthweight infants. Acta Paediatr. 2004; 93(12):1563–1568. PMID:
15841762.
33. Horbar JD, Ehrenkranz RA, Badger GJ, Edwards EM, Morrow KA, Soll RF, et al. Weight growth velocity and postnatal growth failure in infants 501 to 1500 grams: 2000–2013. Pediatrics. 2015; 136(1):e84–e92. PMID:
26101360.

34. Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009; 123(1):e101–e109. PMID:
19117831.

35. Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011; 128(4):e899–e906. PMID:
21949135.

36. Maruyama H, Yonemoto N, Kono Y, Kusuda S, Fujimura M. Neonatal Research Network of Japan. Weight growth velocity and neurodevelopmental outcomes in extremely low birth weight infants. PLoS One. 2015; 10(9):e0139014. PMID:
26402326.

37. Ardila A, Pineda D, Rosselli M. Correlation between intelligence test scores and executive function measures. Arch Clin Neuropsychol. 2000; 15(1):31–36. PMID:
14590565.

38. Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009; 15(3):331–343. PMID:
19402919.

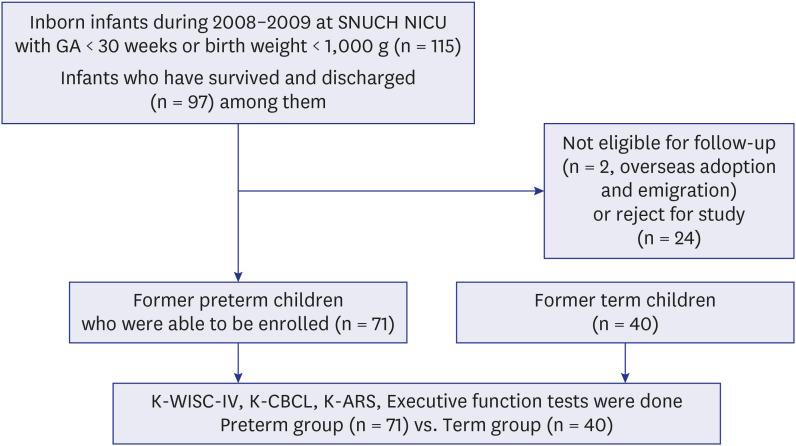
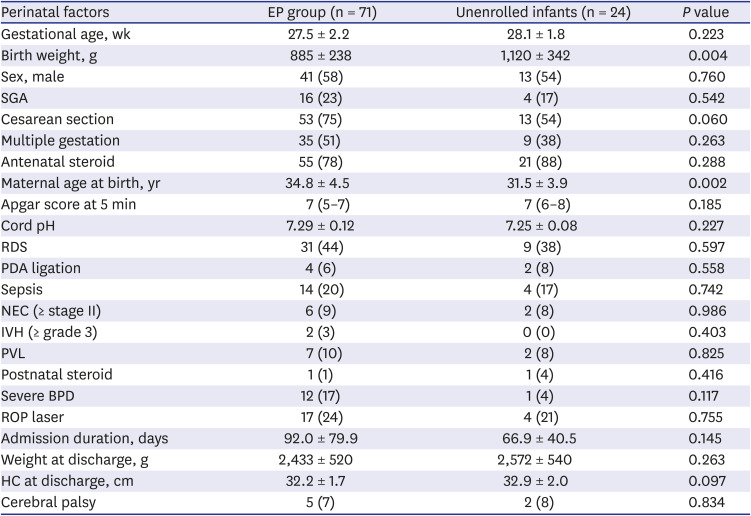
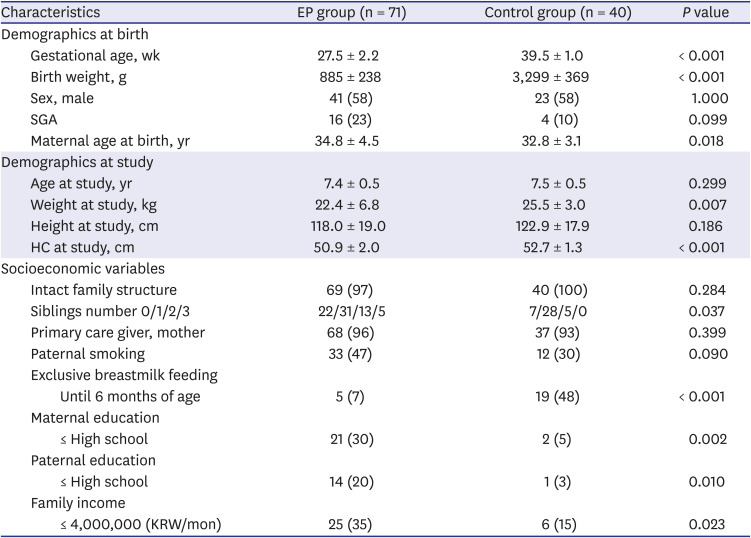
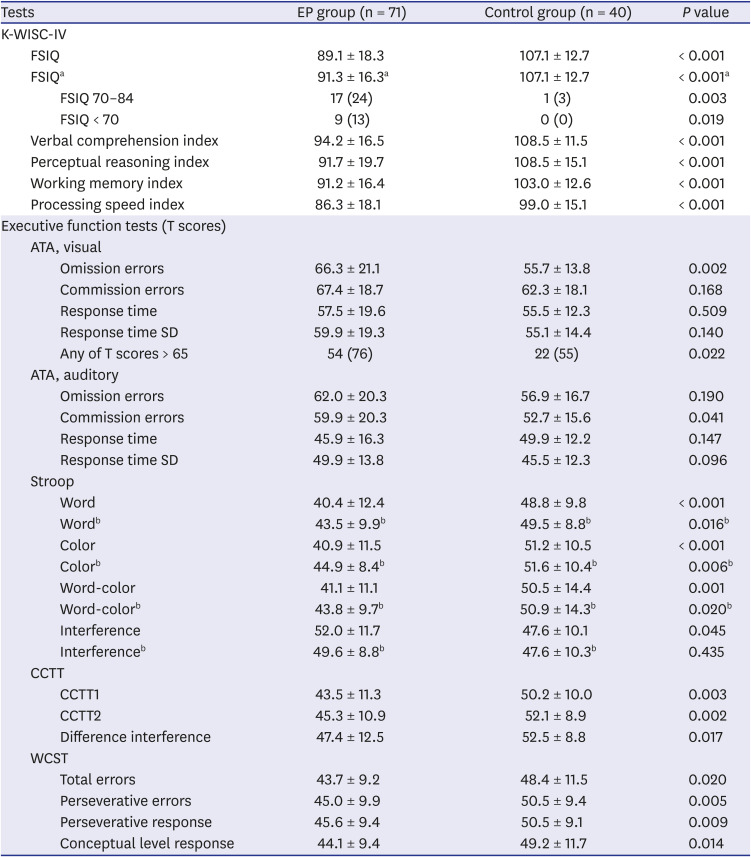




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download