INTRODUCTION
Germline mutations in
BRCA1 or
BRCA2, the most commonly detected mutations in hereditary breast and ovarian cancer, are found in up to 15–20% of unselected epithelial ovarian cancer (EOC) patients.
12 Based on this relatively high prevalence of
BRCA-mutated EOC,
BRCA gene testing is recommended in all patients diagnosed with EOC at any age.
3 Conventionally, knowing the
BRCA mutation status has been important for EOC patients and their family members in terms of assessing the genetic risk of hereditary cancer. However, the development of poly (ADP-ribose) polymerase inhibitors (PARPi) and their demonstrated clinical benefits in
BRCA-mutated EOC patients have drawn greater attention to genetic testing for
BRCA1 and
BRCA2 as a companion diagnostic test for the usage of PARPi, and the prescription number of
BRCA gene testing has markedly increased in the last decade along with the health insurance coverage.
456
The cumulative risk of breast cancer up to age 70–80 years in
BRCA1 and
BRCA2 mutation carriers was reported to be 72% for
BRCA1 and 66.3–69% for
BRCA2 mutation.
78 Due to the high risk of breast cancer development especially at an early age in these carriers, current guidelines recommend annual mammography and breast MRI starting at 30 years of age.
3
However, despite the increased performance of
BRCA gene testing, the uptake of breast cancer risk reduction strategies for
BRCA mutation carriers, including breast cancer screening, has been suboptimal with significant differences country by country.
910 Although there have been a few studies assessing the frequency of risk-reducing salpingo-oophorectomy among
BRCA mutation carriers, the state of breast cancer screening uptake and cascade testing among Korean women with
BRCA mutations has not been evaluated up to date.
1112 Therefore, this study aimed to assess the rate of germline BRCA gene tests in EOC patients and uptake of post-test risk management strategies, including breast cancer surveillance and cascade testing on family members in
BRCA1/2-mutated patients.
Go to :

METHODS
The institutional databases were searched to identify patients who were diagnosed with epithelial ovarian, fallopian tube, or primary peritoneal cancer between January 2009 and December 2019 in two academic hospitals (Ewha Womans University Medical Center [EUMC] and Seoul National University Hospital [SNUH]). Exclusion criteria include non-EOC cases, insufficient data, and patients diagnosed outside the study period. Retrospective review of electronic medical records was performed to collect clinico-pathologic variables, which include age at diagnosis, histologic type, stage, performance of germline BRCA gene test and its results, and conduct of breast cancer screening tests. If annual mammography with or without breast ultrasonography was performed among women aged ≥ 40 years, it was considered that regular breast cancer surveillance was done. For women aged < 40 years, annual breast ultrasonography with or without mammography was regarded as regular breast screening test. If breast cancer screening test was performed only one time during the follow-up period, it was not counted as performance of regular breast cancer surveillance.
Recommendation and performance of cascade testing for family members were also investigated.
Statistical analysis was performed using SPSS for Windows (version 20.0; SPSS Inc., Chicago, IL, USA). In most of the cases, descriptive statistical analysis was performed. Either χ2 test or Fisher's exact test was used when comparing categorical variables. Two-sided P value < 0.05 was considered significant.
Ethics statement
Institutional Review Board (IRB) approval of a waiver of informed consent (IRB No. 2020-08-013 [EUMC] and No. 2008-097-1149 [SNUH]) was obtained.
Go to :

RESULTS
Between 2009 and 2019, a total of 840 patients who were diagnosed with epithelial ovarian, fallopian tube, or primary peritoneal cancer were identified, of which 233 patients were from EUMC and 607 patients were from SNUH. About two thirds of the patients were diagnosed with stage III/IV disease and the most common histologic type was serous type (69.2%,
Table 1).
Table 1
Patient characteristics
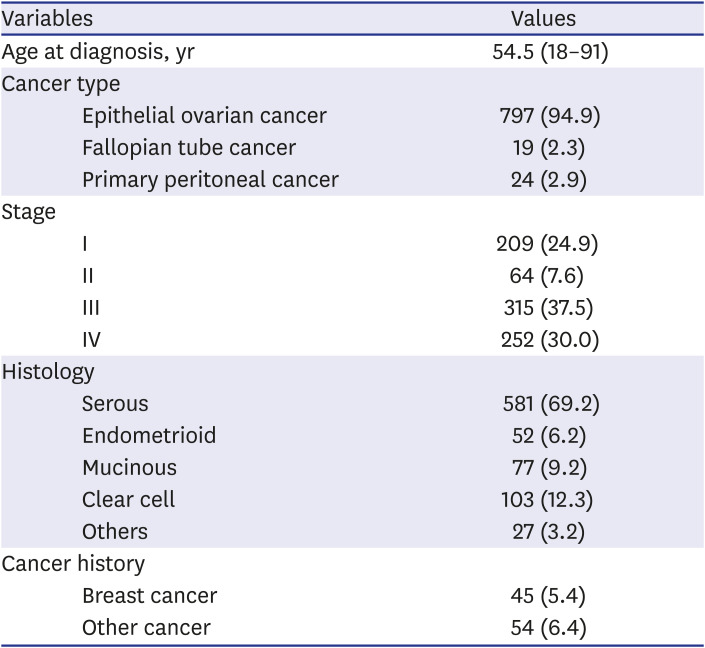
|
Variables |
Values |
|
Age at diagnosis, yr |
54.5 (18–91) |
|
Cancer type |
|
|
Epithelial ovarian cancer |
797 (94.9) |
|
Fallopian tube cancer |
19 (2.3) |
|
Primary peritoneal cancer |
24 (2.9) |
|
Stage |
|
|
I |
209 (24.9) |
|
II |
64 (7.6) |
|
III |
315 (37.5) |
|
IV |
252 (30.0) |
|
Histology |
|
|
Serous |
581 (69.2) |
|
Endometrioid |
52 (6.2) |
|
Mucinous |
77 (9.2) |
|
Clear cell |
103 (12.3) |
|
Others |
27 (3.2) |
|
Cancer history |
|
|
Breast cancer |
45 (5.4) |
|
Other cancer |
54 (6.4) |

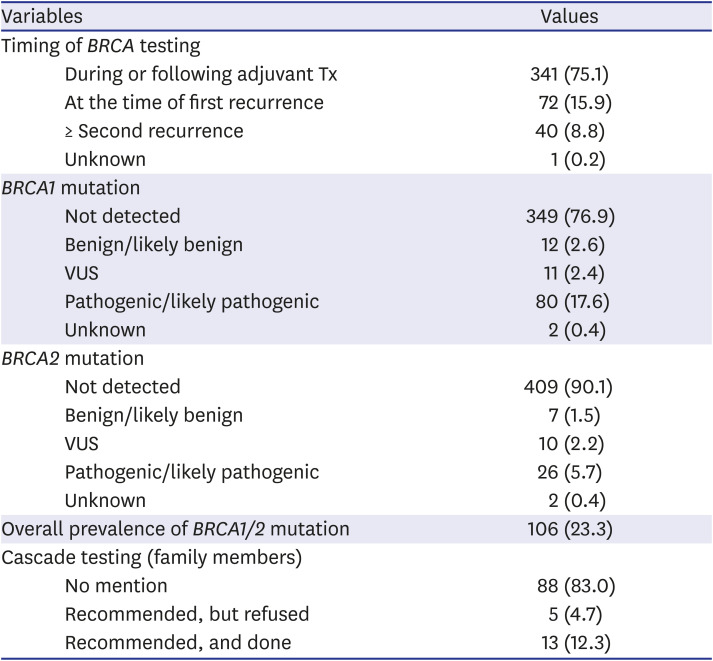
In 454 patients (54.0%), germline
BRCA gene testing was performed. The rate of
BRCA tests has markedly increased from 25.8% in 2009–2012 to 54.2% in 2013–2016 and 62.7% in 2017–2019. The
BRCA gene testing was more frequently performed in stage III/IV disease (62.1%, 352/567) compared to stage I/II disease (37.4%, 102/273;
P < 0.001). Most of the
BRCA gene tests were performed during or following the course of adjuvant chemotherapy after histologic diagnosis was confirmed (75.1%,
Table 2). Among the 454 patients who received
BRCA gene testing, 106 patients (23.3%) were positive for pathogenic/likely pathogenic
BRCA1/2 variants, of which 80 (17.6%) had
BRCA1 and 26 (5.7%) had
BRCA2 mutations. There was no significant difference in the
BRCA1/2 mutation prevalence between the two institutions (data not shown).
Table 2
Timing of BRCA testing and BRCA test results
|
Variables |
Values |
|
Timing of BRCA testing |
|
|
During or following adjuvant Tx |
341 (75.1) |
|
At the time of first recurrence |
72 (15.9) |
|
≥ Second recurrence |
40 (8.8) |
|
Unknown |
1 (0.2) |
|
BRCA1 mutation |
|
|
Not detected |
349 (76.9) |
|
Benign/likely benign |
12 (2.6) |
|
VUS |
11 (2.4) |
|
Pathogenic/likely pathogenic |
80 (17.6) |
|
Unknown |
2 (0.4) |
|
BRCA2 mutation |
|
|
Not detected |
409 (90.1) |
|
Benign/likely benign |
7 (1.5) |
|
VUS |
10 (2.2) |
|
Pathogenic/likely pathogenic |
26 (5.7) |
|
Unknown |
2 (0.4) |
|
Overall prevalence of BRCA1/2 mutation |
106 (23.3) |
|
Cascade testing (family members) |
|
|
No mention |
88 (83.0) |
|
Recommended, but refused |
5 (4.7) |
|
Recommended, and done |
13 (12.3) |

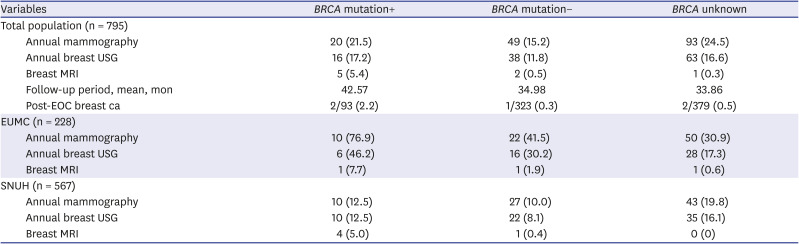
Among the 93 patients with BRCA1/2 mutation without previous personal breast cancer history, 20 patients (21.5%) received annual mammography with or without breast ultrasonography. There were only 2 patients aged < 40 years, and one of these two patients received annual breast cancer surveillance with mammography alone. Breast MRI was performed in five patients, of whom only one patient with BRCA1 pathogenic variant received regular breast MRI for surveillance. And, one patient treated for stage IC EOC was tested to have BRCA1 mutation and received risk-reducing bilateral mastectomy.
There were significant differences in the uptake of breast cancer screening by institution. In EUMC, 76.9% of patients with
BRCA1/2 mutation received annual mammography, which was significantly higher than patients without
BRCA mutation (41.5%,
Table 3). On the other hand, only 12.5% of
BRCA1/2 mutated patients in SNUH received annual mammography.
Table 3
Breast cancer surveillance among patients without history of breast cancer according to the BRCA status
|
Variables |
BRCA mutation+ |
BRCA mutation− |
BRCA unknown |
|
Total population (n = 795) |
|
|
|
|
Annual mammography |
20 (21.5) |
49 (15.2) |
93 (24.5) |
|
Annual breast USG |
16 (17.2) |
38 (11.8) |
63 (16.6) |
|
Breast MRI |
5 (5.4) |
2 (0.5) |
1 (0.3) |
|
Follow-up period, mean, mon |
42.57 |
34.98 |
33.86 |
|
Post-EOC breast ca |
2/93 (2.2) |
1/323 (0.3) |
2/379 (0.5) |
|
EUMC (n = 228) |
|
|
|
|
Annual mammography |
10 (76.9) |
22 (41.5) |
50 (30.9) |
|
Annual breast USG |
6 (46.2) |
16 (30.2) |
28 (17.3) |
|
Breast MRI |
1 (7.7) |
1 (1.9) |
1 (0.6) |
|
SNUH (n = 567) |
|
|
|
|
Annual mammography |
10 (12.5) |
27 (10.0) |
43 (19.8) |
|
Annual breast USG |
10 (12.5) |
22 (8.1) |
35 (16.1) |
|
Breast MRI |
4 (5.0) |
1 (0.4) |
0 (0) |

Among the 106
BRCA1/2-mutated EOC patients, cascade testing on family members was performed only in 13 patients (12.3%,
Table 2). There was no mention or recommendation on family member testing in most of the patients (83.0%). Among the 18 patients who were recommended for the cascade testing, 72.2% received cascade testing. There was also a significant difference in the rate of cascade testing between the two institutions, although the rates in the two institutions were both suboptimal (35.7% in EUMC vs. 8.7% in SNUH;
P = 0.013).
During the median follow-up period of 42.6 months, two patients, one patient from each institution, were diagnosed with breast cancer before
BRCA gene testing was done. When counting the prescription number of
BRCA gene testing in the two institutions by year, there was a marked increase in the number of
BRCA gene testing, especially after 2016, and the number of
BRCA1/2 mutation carriers has also increased proportionately (
Fig. 1). However, the rate of performance of breast cancer screening test has not increased accordingly, particularly in patients with
BRCA1/2 mutation.
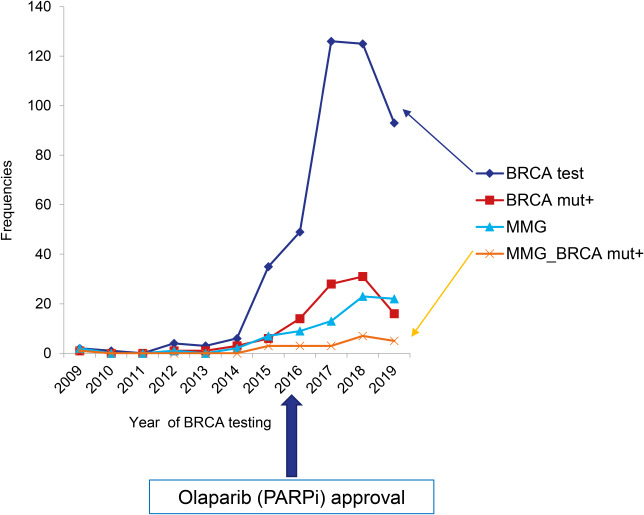
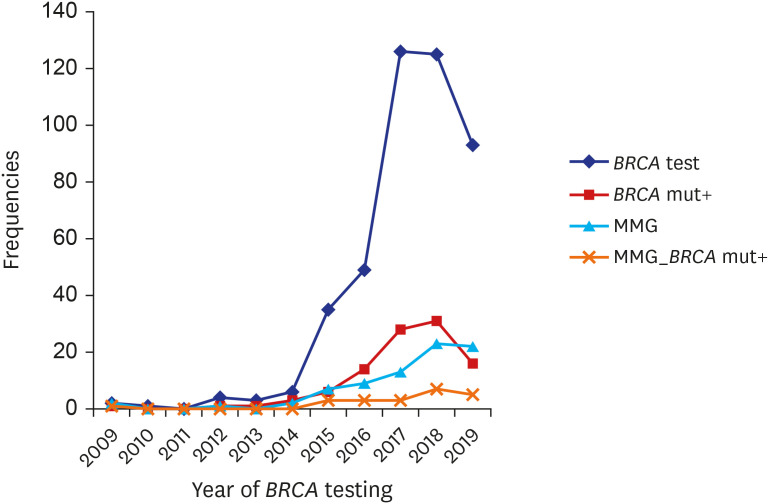 | Fig. 1
Annual trend of BRCA gene testing prescription (♦), BRCA1/2 mutation carriers (▪), and breast cancer surveillance with mammography in EOC patients as a whole (▴) and EOC patients with BRCA mutations (x).
EOC = epithelial ovarian cancer, MMG = mammography.

|
Go to :

DISCUSSION
The present study evaluated the rate of germline BRCA gene tests among EOC patients and the uptake of breast cancer screening strategies and cascade testing among BRCA1/2-mutated patients from two academic institutions in Korea. Although there has been a marked increase in the number of BRCA gene testing, the rates of breast cancer surveillance and cascade testing were low at only 21.5% and 12.3%, respectively.
Germline
BRCA gene test is indicated in every EOC case at any age, and the decision of carrying out the genetic tests is usually made by the attending physicians. Although there was a survey study reporting that pre/post-test counseling and recommendation for risk-reducing surgery were appropriately provided by gynecologic oncologists, the study result was somewhat biased since the survey was done only on members participating in the hereditary gynecologic cancer symposium.
13 Ultimately, the decision on the evaluation of hereditary cancer risk and the implementation of the post-test risk management strategies are largely dependent on the individual physician's interest and awareness.
BRCA gene testing has substantially expanded especially after 2016, when Olaparib, one type of PARPi, was first approved in Korea as a maintenance therapy for
BRCA-mutated, platinum-sensitive recurrent EOC (
Fig. 1). Although the number of
BRCA1/2 mutation carriers has increased accordingly, the rate of breast cancer screening among EOC patients carrying
BRCA mutations has remained low. Also, the cascade testing for family members was recommended in only 17% of the patients with
BRCA1/2 mutations. These findings suggest that
BRCA gene testing itself has gained clinical interest as a companion diagnostic test for the usage of PARPi, but the knowledge of
BRCA status derived from the test did not lead to appropriate follow-up measures. Also, the observation that
BRCA gene testing was more frequently performed in advanced stage (62.1% in stage III/IV vs. 37.4% in stage I/II) suggested that the main purpose of the genetic tests might be to find candidates for PARPi, rather than to evaluate the hereditary cancer risk.
Since women with
BRCA1 or
BRCA2 mutation have elevated risks of
BRCA-related cancer, including breast cancer, the National Comprehensive Cancer Network guidelines recommend beginning breast cancer surveillance with clinical breast exam at age 25 years, annual breast MRI at age 25–29 years, and annual mammography at age 30 years.
3 The sensitivity of MRI has been reported to be consistently higher than that of mammography, and the majority of breast cancers detected by MRI were early-stage cancers.
14 There were also some preliminary studies reporting that MRI screening might offer survival benefit especially in
BRCA2 mutation carriers.
15 Due to the potential risk of radiation exposure and lesser sensitivity of breast cancer detection by mammography, current guidelines prefer screening with MRI over mammography, especially in younger women at a high risk of breast cancer. In Korea, however, breast MRI screening for
BRCA mutation carriers is not yet covered by national health insurance. In our study, only one patient received annual breast MRI for routine surveillance. To improve the uptake of breast MRI screening in
BRCA mutation carriers, insurance issues need to be discussed further.
Some researchers reported that the risk of breast cancer following EOC in
BRCA mutation carriers was low (8.3–8.9%) with the median time of 50 months from diagnosis of EOC to diagnosis of breast cancer, and that the survival was dominated by EOC-related mortality.
1617 Based on these findings, some argue that breast cancer surveillance in
BRCA-related EOC patients might not be as important as in unaffected
BRCA mutation carriers. However, similar mortality rates between
BRCA-mutated EOC patients with and without breast cancer diagnosis following EOC may be attributed to exceedingly poor survival outcomes of advanced EOC and early detection of early-stage breast cancer. It does not mean that breast cancer surveillance could be overlooked, especially in early-stage EOC patients having good prognosis. In our study, the rate of annual mammography was similarly low in stage I/II EOC (20.0%, 2/10), compared to stage III/IV disease (21.7%, 18/83;
P = 0.092). Efforts to increase the awareness of importance of breast cancer surveillance in early-stage EOC patients at least are needed.
However, prophylactic mastectomy in BRCA-mutated EOC patients needs to be discussed with caution since aforementioned studies demonstrated that survival outcomes of EOC patients with metachronous breast cancer did not differ from those of EOC patients without breast cancer. Risk-reducing mastectomy, which is now covered by national health insurance for BRCA mutation carriers, may be considered in a limited number of early-stage EOC patients with BRCA mutations, for whom long-term survival can be expected.
In our study, the cascade testing for family members was performed in only 12.3% of the patients with BRCA1/2 mutations. Among the patients who were recommended for the cascade testing by their physicians or specialized geneticists, however, 72.2% received cascade testing. And, although the rate was still low, patients treated at EUMC where hereditary cancer clinic has been run by department of laboratory medicine since 2017 more likely received cascade testing (5/14 in EUMC vs. 8/92 in SNUH), and four of the five patients who performed the family member testing received genetic counseling at the hereditary cancer clinic. Based on these findings, it was suggested that performance of cascade testing might be affected by physicians' attitudes and post-test counseling. The knowledge of the BRCA mutation status of EOC patients is important for their family members as well in that it can afford opportunities for prevention of BRCA-related cancers in unaffected family members with BRCA mutations. For patients and their physicians, more education on the importance of post-test counseling, including risk-reducing strategies and cascade testing, is needed. In addition to the education, appropriate allocation of medical charge for genetic counseling is essential. At present, there is no charge for genetic counseling, which makes it difficult to give sufficient genetic counseling to the patients who received hereditary cancer-related genetic tests.
There are several limitations to our study. First, our study may not represent the nationwide uptake of breast cancer screening in the population of BRCA-mutated EOC patients since only EOC patients from two hospitals were included in the study population. In addition, the possibility of patients receiving breast cancer surveillance at other institutions was not investigated since the conduct of breast cancer screening was retrospectively reviewed based on medical records. However, higher rates of breast cancer surveillance and cascade testing in the institution where hereditary cancer clinic is settled are remarkable. To develop more comprehensive insight on the trend in the uptake of breast cancer surveillance and cascade testing among EOC patients with BRCA mutations, nationwide survey using national health insurance data is needed in the future.
Overall, the uptake of breast cancer screening among Korean EOC patients with BRCA1/2 mutations is still not optimal (21.5% on annual mammography), and the recommendation of cascade testing for family members is inadequately done (17%). Strategies to increase its uptake and more efforts on educating physicians and patients about the importance of risk-reducing strategies and cascade testing after positive BRCA gene testing results as well as applying for appropriate coverage of national health insurance on risk-reducing strategies are needed.
Go to :