INTRODUCTION
METHODS
Cell cultures and differentiation of osteoblasts and osteoclasts
AGEs treatment
TRAP activity assay
Alkaline phosphatase (ALP) and bone nodule formation assays
RNA extraction and gene expression analysis
Table 1
Primer sequences and conditions for reverse transcription polymerase chain reaction
Western blot analysis
Statistical analysis
Ethics statement
RESULTS
Receptor for AGEs (RAGE) expression on cells
 | Fig. 1RAGE expression in MC3T3-E1 cells, BMM cells, and marrow-derived MNCs using reverse transcription polymerase chain reaction. RAGE was expressed on all cells. The grouping of gels/blots cropped from different parts of the same gel.RAGE = receptor for advanced glycated end products, BMM = bone marrow mononuclear, MNC = multinucleated cell.
|
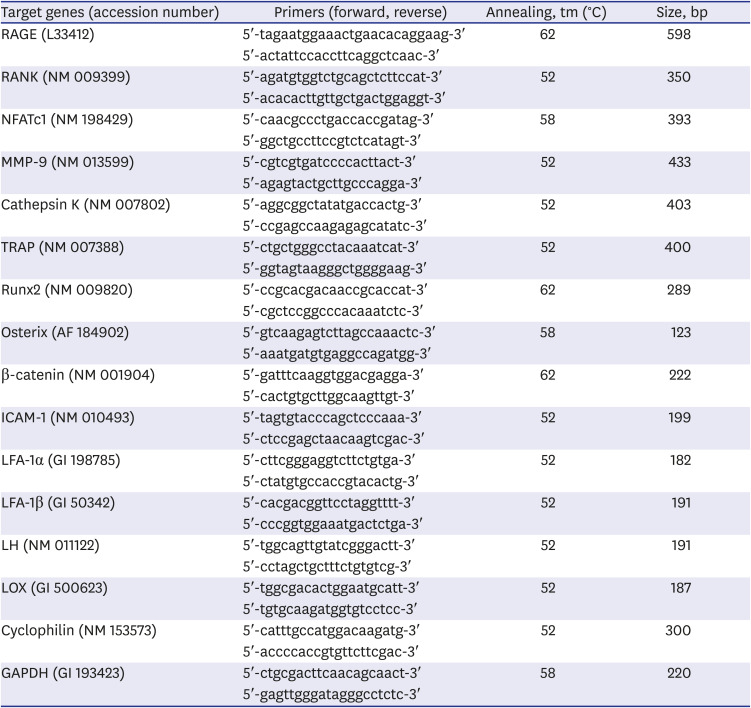
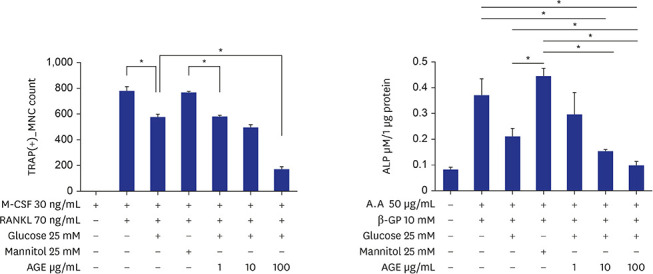
Inhibitory effects of AGEs on osteoclast and osteoblast differentiation
 | Fig. 2Effects of AGEs on osteoclast and osteoblast differentiation. (A) TRAP-positive MNCs in RANKL-induced marrow macrophage cell cultures. Bone marrow mononuclear cells were incubated for three days with 30 ng/mL M-CSF, 70 ng/mL RANKL, 25 mM glucose, 25 mM mannitol, and 1, 10, or 100 ug/mL of AGEs (*P < 0.05). (B) ALP activities in MC3T3-E1 cell cultures. MC3TC-E1 cells were incubated for eight days with 50 ug/mL ascorbic acid, 10 mM β-GP, 25 mM glucose, 25 mM mannitol, and 1, 10, or 100 ug/mL of AGEs (*P < 0.05, **P < 0.01, ***P < 0.001). (C) Bone nodule formations in MC3T3-E1 cell cultures. MC3TC-E1 cells were incubated in 24-well plates (5,000 cells/well) for 14 days with 50 ug/mL ascorbic acid, 10 mM β-GP, 25 mM glucose, 25 mM mannitol, and 1, 10, or 100 ug/mL of AGEs (*P < 0.05, **P < 0.01).TRAP = tartrate-resistant acid phosphatase, MNC = multinucleated cell, M-CSF = macrophage colony-stimulating factor, RANKL = receptor activator of nuclear factor-κB ligand, AGE = advanced glycation end product, ALP = alkaline phosphatase.
|
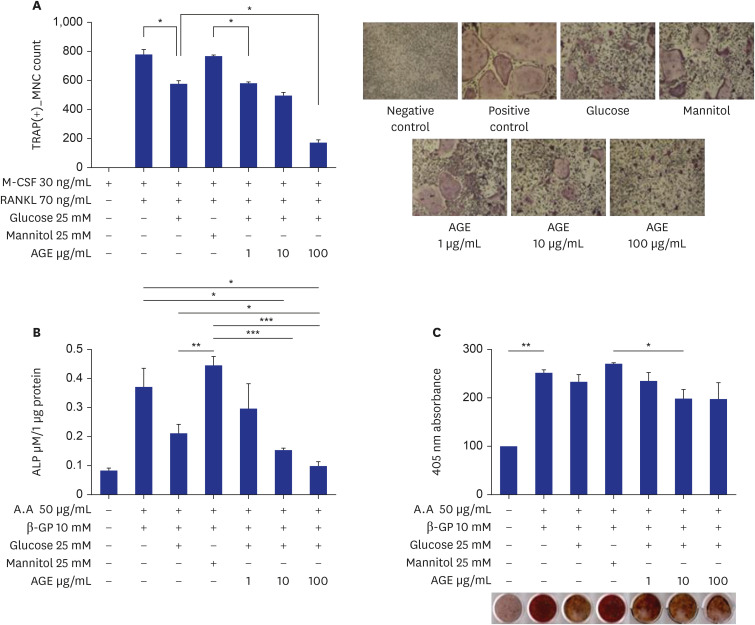
Effects of AGEs on expression of osteoclast-specific genes and mitogen-activated protein kinase (MAPK) signaling pathways in RANKL-induced marrow cells
 | Fig. 3Effects of AGEs on osteoclast-specific genes (A) and activation of the mitogen activated protein kinase signaling pathway (B) in RANKL-induced marrow macrophages. The osteoclast-specific genes such as RANK, TRAP, MMP-9, cathepsin K, and NFATc1 were measured by reverse transcription polymerase chain reaction. JNK, p38, ERK, AKT and β-actin were measured by western blots. The grouping of gels/blots cropped from different parts of the same gel.NC = negative control, PC = positive control, G = 25 mM glucose, M = 25 mM mannitol, AGE = advanced glycated end product, RANKL = receptor activator of nuclear factor-κB ligand, TRAP = tartrate-resistant acid phosphatase, MMP-9 = matrix metalloproteinases-9, NFATc1 = nuclear factor of activated T-cells cytoplasmic 1.
|
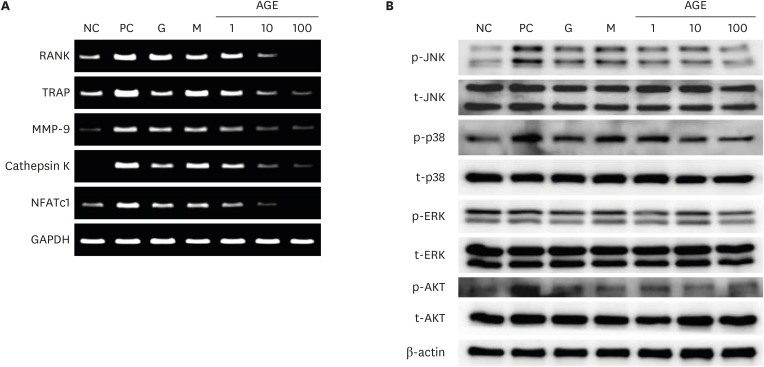
Effects of AGEs on ICAM-1 and LFA-1 expression
 | Fig. 4Effects of AGEs on ICAM-1 and LFA-1 expression in marrow macrophages. Expressions of ICAM-1, LFA-1α, and LFA-1β genes were measured by reverse transcription polymerase chain reaction. The grouping of gels/blots cropped from different parts of the same gel.NC=negative control, PC=positive control, G=25mM glucose, M=25mM mannitol, AGE = advanced glycated end product, ICAM-1 = intercellular adhesion molecule 1, LFA-1 = lymphocyte function-associated antigen 1.
|
Effects of AGEs on expression of osteoblast-specific genes, LH, and LOX genes in MC3T3-E1 cells
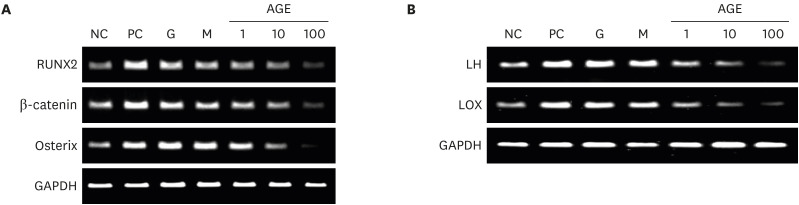 | Fig. 5Effects of AGEs on osteoblast-specific genes (A) and expression of LH and LOX genes (B) in MC3T3-E1 cells. The grouping of gels/blots cropped from different parts of the same gel. The osteoblast-specific genes, LH and LOX genes were measured by reverse transcription polymerase chain reaction.NC = negative control, PC = positive control, G = 25 mM glucose, M = 25 mM mannitol, AGE = advanced glycated end product, LH = lysyl hydroxylase, LOX = lysyl oxidase.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download