INTRODUCTION
Probiotics are defined as live microorganisms that confer beneficial health effects to the host (1, 2, 3). Most probiotic microorganisms are lactic acid bacteria (LAB), such as Lactobacillus sp., Leuconostoc sp., Lactococcus sp., Enterococcus sp., and Weissella sp. (4). The effects of probiotics on animals and humans have been studied intensively, and many reports suggest the use of probiotics as preventive or therapeutic agents for various pathological conditions. For instance, some probiotics have been shown to improve hypertension (5) and diarrhea induced by antibiotics or rotavirus (6, 7), prevent cancer (8, 9), reduce cholesterol levels (10, 11), or alleviate intestinal problems, such as irritable bowel syndrome and inflammatory bowel disease (12, 13). With the increased prevalence of various diseases related to the immune response, much interest is focused on the prevention or treatment of diseases through the immunomodulatory effects of LAB (14).
LAB are gram-positive, lactic acid-producing, non-spore-forming cocci or rod-shaped bacteria, usually found in fermented foods and dairy products (15, 16). Weissella sp. were classified as LAB in 1993 (17). Bacteria in this genus are often found in the gastrointestinal tract of humans or animals, as well as in fermented foods, such as fermented sausages (18, 19). Weissella cibaria has been isolated from Korean fermented food, various other foods, and human and animal feces (20). Several studies have investigated the probiotic potential of the Weissella genus and some functional properties relevant to their use as probiotics, particularly their ability to enhance immune responses by inducing the generation of inflammatory mediators (20, 21). Notably, W. cibaria produces weissellicin, an antibacterial substance (21), with an anticancer effect in colorectal cancer (22) and an antiviral effect against avian influenza virus (23). In addition, the W. cibaria strain JW15 (JW15) isolated from kimchi, a Korean traditional fermented food, possesses immune-stimulatory activity and induces pro-inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor-alpha (TNF-α), as well as nitric oxide, in macrophages (24). These findings suggest that W. cibaria could be developed as a probiotic to enhance the immune responses to infectious diseases or cancers.
Natural killer (NK) cells are granulocytes with an important role in initiating the innate immune response (25). NK cells recognize and kill tumor and virus-infected cells without prior specific sensitization (26). NK cells exhibit cytotoxic effects by recognizing target cells based on the absence of major histocompatibility complex class I (MHC I) molecules (27, 28). To evade immune detection and elimination, viruses often down-regulate these molecules, and cancer cells express low levels of MHC I molecules. However, both are preferentially targeted and removed by NK cells (29). NK cell cytotoxicity is mediated by the binding of death receptors or the release of cytolytic granules stored in the cytoplasm upon target cell recognition, or both (30, 31). The granules contain perforin and granzymes, pore-forming protein and serine proteases, respectively. Released perforin forms pores in the target cell membrane, leading to the entry of granzymes into the cells, which, in turn, initiate apoptosis (32). NK cells also produce type II interferon (IFN), IFN-γ, which enhances NK cell activity by positive feedback loop and activate macrophages (33, 34). IFN-γ plays an important role in both adaptive and innate immunity. Therefore, it is plausible to suggest that enhancing NK cell activity is an attractive strategy to prevent or treat viral infection and cancer.
Various plant molecules or extracts can activate NK cells (35, 36). In addition, some LAB strains activate NK cell activity in vitro and in vivo. For example, Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 increased NK cell activity and antiviral activity against the influenza virus (37, 38). Bifidobacterium lactis HN019 enhanced tumoricidal activity of NK cells (39). However, it is not clear whether the immunostimulatory effects of JW15 are mediated by NK cell activation because previous studies used a mixture of lymphocytes or macrophages (24). To resolve this dilemma, the present study was designed to evaluate immunoregulatory effect of JW15 on murine NK cell activation and cytotoxicity in vitro and in vivo.
Go to : 
MATERIALS AND METHODS
Bacterial culture
JW15 (KACC 91811P), isolated from kimchi, and Lactobacillus rhamnosus GG (LGG, ATCC 53103) were cultured in Lactobacilli de Man–Rogosa–Sharpe (MRS) broth (BD Biosciences, San Jose, CA, USA) at 200 rpm, 37℃ for 5.5 and 14.5 h, respectively. Bacterial cells were collected by centrifugation at 1,500 × g for 10 min. The resultant cell pellet was washed twice with sterile phosphate-buffered saline (PBS, pH 7.2). Viable bacterial cells were counted on MRS agar plates.
Mice
BALB/c and C57BL/6 male mice were purchased from Samtako, Inc. (Osan-si, Republic of Korea) and were maintained at 22–24℃ and 45–55% relative humidity under a 12 h light/dark cycle. Mice were fed normal chow. All mice experiments were performed according to the guidelines from the Animal Care and Use Committee (ACUC) of Jeonbuk National University (Approval number: CBNU 2019-014).
Splenocytes preparation
Spleens were isolated from BALB/c mice, and single-cell suspensions were prepared by passing through a 40-μm cell strainer (BD Falcon, Bedford, MA, USA). Cells were washed with PBS and treated with red blood cell lysis buffer (eBioscience, USA). Then, cells were resuspended in Roswell Park Memorial Institute (RPMI) 1640 medium (GE Healthcare Life Sciences, USA) supplemented with 2.05 mM L-glutamine, 10% (v/v) heat-inactivated fetal bovine serum (FBS; Capricorn Scientific, Germany) and 1% (v/v) penicillin–streptomycin (10,000 U/mL penicillin, 10,000 μg/mL streptomycin; Gibco, USA).
Isolation of NK cells
NK cells were isolated from the splenocytes of BALB/c mice using the MagniSort Mouse NK cell Enrichment Kit (eBioscience), in accordance with the manufacturer’s instructions. NK cells were cultured in RPMI 1640 medium (GE Healthcare Life Sciences) supplemented with 2.05 mM L-glutamine, 10% (v/v) heat-inactivated FBS (Capricorn Scientific) and 1% (v/v) penicillin–streptomycin (10,000 U/mL penicillin, 10,000 μg/mL streptomycin; Gibco).
NK cell cytotoxicity assay
Splenoctyes or NK cells were seeded in a 96-well plate and co-cultured with Yac-1 cells (NK sensitive‐target cell) stained with carboxyfluorescein succinimidyl ester (CFSE; eBioscience) at an effector cell:target cell ratio of 10:1. After 4 h, cells were stained with propidium iodide (PI) staining solution (eBioscience) and dead cells (CFSE+ and PI+) analyzed using a BD Acurri C6 flow cytometer (BD Biosciences).
Flow cytometry analysis of NK cell activation markers
Cells were collected and washed with FACS buffer (3% HI-FBS and 0.02% sodium azide in PBS). Then, single-cell suspensions were stained with anti-mouse CD3 (PerCP/Cy5.5, BioLegend, USA), CD49b (APC, BioLegend), CD69 (FITC, eBioscience), or NKG2D/CD314 (PE, BioLegend) antibodies in FACS buffer and incubated for 30 min on ice in the dark, followed by flow cytometry analysis. For intracellular cytokine staining, cells were restimulated with PMA/ionomycin (eBioscience) and brefeldin A (eBioscience) and incubated at 37℃ for 4 h. After cell surface staining, cells were incubated with IC Fixation Buffer (eBioscience) for 30 min and washed using 1X Permeabilization Buffer (eBioscience). Cells were stained with anti-mouse granzyme B (FITC, eBioscience), perforin (PE, eBioscience), or IFN-γ (FITC, BioLegend) antibodies in 1X Permeabilization Buffer for 30 min. Cells were resuspended in FACS buffer, and the results were detected and analyzed by flow cytometry.
Enzyme-linked immunosorbent assay (ELISA)
Cell culture supernatants were harvested from EPS treatment experiments and used to determine cytokine levels using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. The ELISA kits used were mouse TNF-α, IL-12 (BioLegend), IL-6, IL-17, and IFN-γ (eBioscience).
Isolation and purification of EPS from JW15 culture
EPS was isolated from JW15, as described in previous study (40), Briefly, single colony was inoculated in sucrose broth (1% tryptone, 0.5% yeast extract, 0.5% dipotassium phosphate, 0.5% diammonium citrate, 5% sucrose, pH 7.0) and incubated at 37℃ in a shaking incubator for 48 h. Afterward, the sucrose broth was added with 4% (w/v) trichloroacetic acid (Sigma-Aldrich, St. Louis, MO, USA) and incubated at 4℃ for 2 h. After centrifugation (10,000 × g, 4℃, 25 min), the supernatant was collected and precipitated with two volumes of cold 95% ethanol at 4℃ overnight, then centrifugated (10,000 × g, 4℃, 25 min). The resultant pellet was resuspended in triple distilled water. It was named as the crude EPS solution and treated with 50 μg/mL of DNase Ⅰ (Sigma-Aldrich) and RNase A (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 37℃ for 6 h, followed by 200 μg/mL of proteinase K (GeneAll, Seoul, Republic of Korea) under the same condition. The solution was mixed with two volumes of chilled 95% ethanol and precipitated at 4℃ overnight. The EPS pellet was collected by centrifugation (10,000 × g, 4℃, 25 min), washed with 70% ethanol, and re-centrifuged. After the supernatant was removed, the purified EPS pellet was dissolved in triple distilled water and lyophilized.
Oral administration of JW15 to mice
Eight-week-old C57BL/6 male mice were divided into three groups and administered 100 μL of saline, live JW15, or live LGG (1.0 × 109) once daily via oral gavage. After 14 days, all mice were sacrificed, and the spleens were collected for NK cell activity evaluation.
Statistical analysis
All experimental data are expressed as means ± standard error of the means (SEM) of triplicate samples. Statistical analysis involved unpaired t-tests or a one-way ANOVA at a significance level of p < 0.05 using GraphPad PRISM® (GraphPad Software, Inc., USA).
Go to : 
RESULTS
JW15 activates the cytotoxic activity of splenocytes by activating NK cells
To evaluate the effect of live JW15 on NK cells activity, splenocytes were treated with JW15 at 1:0.1, 1:1, or 1:5 ratio for 20–24 h and tested for cytotoxicity against Yac-1 cells, as target cells. LGG is a well-known probiotic bacterium that was used in this study to compare with JW15. As shown in Fig. 1A, the cytotoxic activity was significantly increased as the ratio of live JW15 to splenocytes was increased (p < 0.01, p < 0.001). A similar effect on cytotoxicity was observed for LGG (p < 0.05, p < 0.01). Although these results suggest that live JW15 stimulates the cytotoxicity of immune cells included in splenocytes, it is unclear whether enhanced cytotoxicity of splenocytes by JW15 treatment is mediated by NK cell activation. To determine whether JW15 is able to activate NK cells, IFN-γ production and cell surface expression of activation markers and granules were measured by flow cytometry. When splenocytes were gated on NK cells (CD3- and CD49b+), the level of CD69 was significantly up-regulated by JW15 and LGG, indicating NK cell activation (Fig. 1B). Cell surface expression of NKG2D, an activating cell surface receptor, was not affected by JW15 stimulation but was down-regulated by LGG (Fig. 1C). Next, we analyzed the intracellular levels of perforin and granzyme, both major components of cytolytic granules of NK cells. JW15 treatment of splenocytes at 1:5 ratio tended to increase granzyme B, albeit without statistical significance, whereas significantly higher perforin levels were detected in JW15-treated NK cells (Fig. 1D,E). Activated NK cells produce IFN-γ. When the frequency of IFN-γ-producing cells was evaluated, it was found that JW15 induced IFN-γ-producing NK cells at higher frequencies compared to LGG, especially at lower ratios (Fig. 1F). These results indicate that JW15 might increase cytotoxicity against Yac-1 cells via NK cell activation.
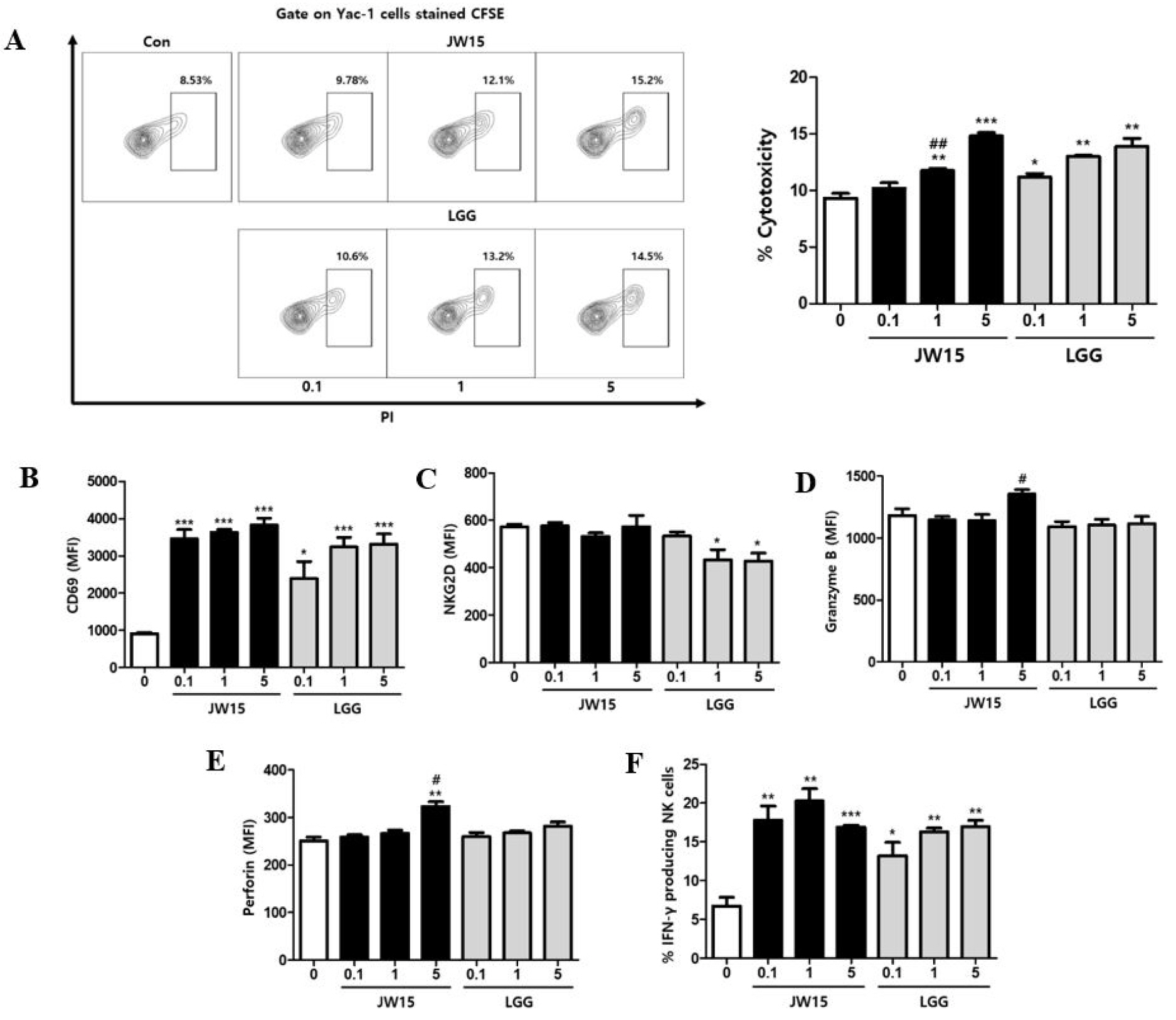 | Fig. 1Effect of JW15 on cytotoxic activity of splenocytes and NK cell activation. Splenocytes of BALB/c mice were treated with live JW15 or LGG at 1:0.1, 1:1, and 1:5 ratio for 20–24 h. (A) Cells were co-cultured with Yac-1 cells stained with CFSE at a 1:10 ratio (Yac-1 cells:splenocytes) for 4 h. Then, cells were stained with PI and detected by flow cytometry. (B–F) Splenocytes were stained with antibodies against CD3, CD49b, CD69, NKG2D, granzyme B, perforin, and IFN-γ and detected by flow cytometry. Cells were gated on CD3-CD49b+ NK cells, and MFI or percentage of each staining is shown. These experiments were repeated three times independently. Results are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group. #p < 0.05, ##p < 0.01 comparison between JW15 and LGG. |
JW15 directly activates NK cells
Although JW15-treated splenocytes showed increased NK cell activation and cytotoxicity, it was still unclear if JW15 acted directly on NK cells. To assess the direct effect of JW15 on NK cells, we first examined whether JW15 affects tumor cell killing by NK cell-mediated cytotoxicity in vitro. NK cells were purified from splenocytes and stimulated with either JW15 or LGG to analyze the cytolytic activity against Yac-1 cells. Compared to untreated cells, JW15 significantly enhanced NK cell cytotoxicity against Yac-1 cells in a dose-dependent manner, unlike LGG, which did not affect the cytotoxic activity of NK cells, even at the highest ratio (Fig. 2A). Next, we examined whether JW15 up-regulated the levels of intracellular granzyme B and perforin. As shown in Fig. 2B,C, JW15-treated NK cells possessed higher levels of granzyme B and perforin in cytoplasm than the non-treated cells. By contrast, LGG treatment did not affect the intracellular levels of granzyme B. Similarly, JW15 augmented the frequencies of NK cells producing IFN-γ by significant amounts, but LGG did not (Fig. 2D). Collectively, these results suggest that JW15 activates NK cells directly and enhances the cytotoxic activity against tumor cells. Contrarily, LGG acts on NK cells indirectly.
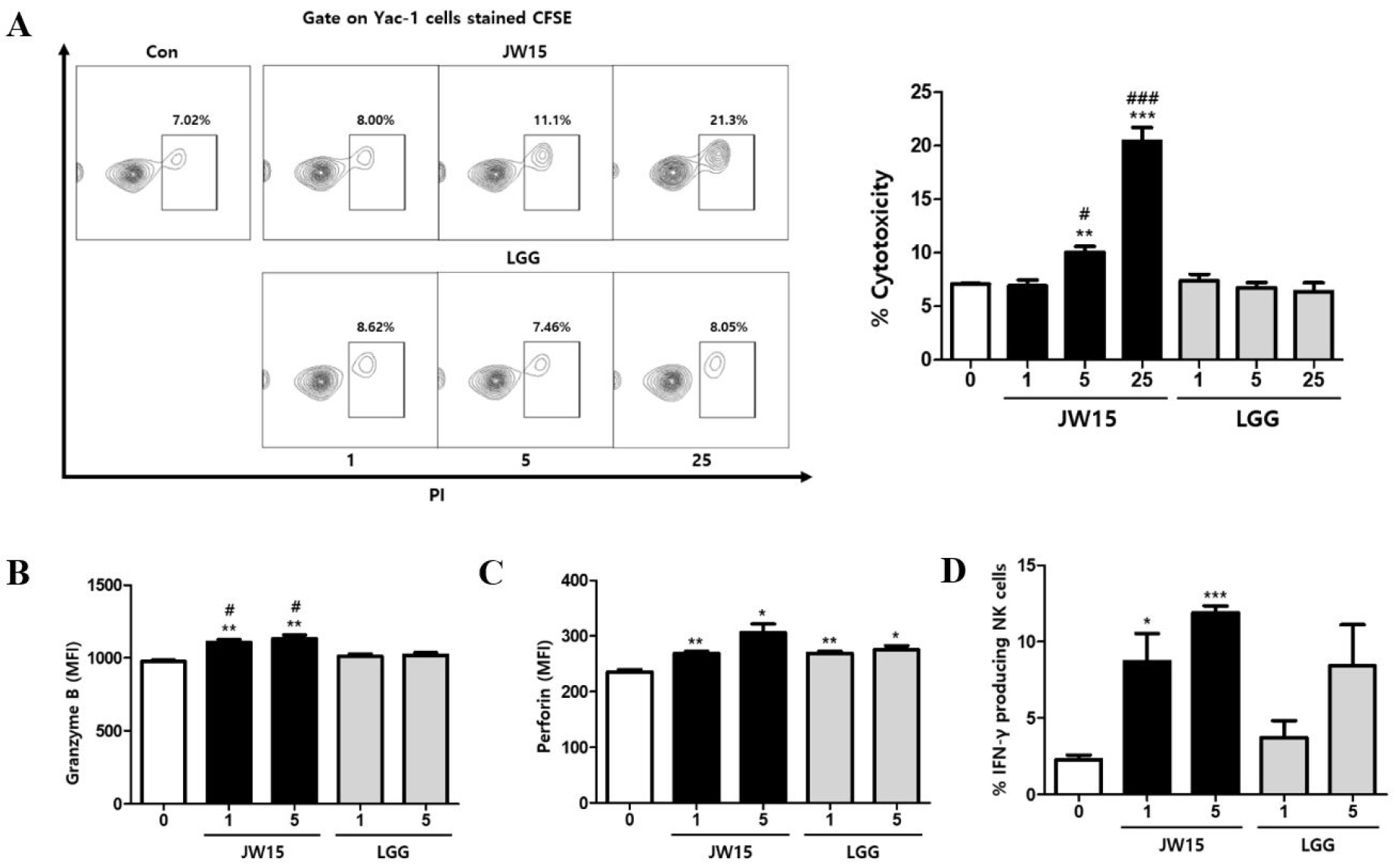 | Fig. 2Activation of NK cells by stimulation with JW15. NK cells isolated from BALB/c mice were treated with live JW15 and LGG in the presence of gentamicin (50 μg/mL). (A) After 20 h, Yac-1 cells labeled with CFSE were co-cultured with NK cells at a 1:10 ratio for 4 h and stained with PI. The frequency of killed Yac-1 cells (PI+ gated on CFSE) was then detected by flow cytometry. (B–D) After 24 h, NK cells were stained with antibodies against CD3, CD49b, CD69, NKG2D, granzyme B, perforin, and IFN-γ and detected by flow cytometry. Cells were gated on CD3-CD49b+ NK cells, and MFI or percentage of each staining is shown. Data are expressed as mean ± SEM of triplicate samples. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group. #p < 0.05, ###p < 0.001 comparison between JW15 and LGG. |
EPS isolated from JW15 activates NK cells indirectly
Various mechanisms by which LAB exert beneficial health effects have been proposed, and EPS was shown to mediate the immune-related function of LAB (41, 42). Here, we investigated whether EPS isolated from JW15 culture could activate immune cells and enhance the cytolytic activity against tumor cells and whether such activity is mediated via NK cell activation. To evaluate if EPS is responsible for the immunomodulatory function of JW15, splenocytes were treated with EPS at various concentrations, and then the cell culture supernatants were analyzed for cytokine secretion. The production of pro-inflammatory cytokines, such as IL-6 and TNF-α, was significantly increased by EPS treatment (Fig. 3A,B). IL-12, mainly produced by dendritic cells and macrophages, was also detected at higher levels in the cell culture supernatant when cells were treated with EPS (Fig. 3C). In addition, EPS treatment up-regulated IFN-γ and IL-17 secretion dose-dependently, and this effect was significant (Fig. 3D,E). Lipopolysaccharide (LPS) was included as a positive control and induced significant levels of cytokines, which were higher than those induced by EPS or comparable with EPS at the highest concentration, except for IL-17. These findings indicate that EPS from JW15 exerts an immunostimulatory effect and suggest that EPS might enhance the cytotoxic activity of NK cells against tumor cells. To test this idea, we performed the NK cell cytotoxicity assay using splenocytes treated with EPS as effector cells. As a result, EPS treatment potentiated the cytotoxicity of splenocytes up to 8.29% compared to the untreated control (Fig. 4A). NK cell activation by EPS was further confirmed by up-regulated expression of CD69, perforin and IFN-γ (Fig. 4B–F). Collectively, these results provide promising evidence that EPS mediates the immune-stimulatory activity of JW15 and plays a role in the NK cell-mediated cytotoxic activity of JW15.
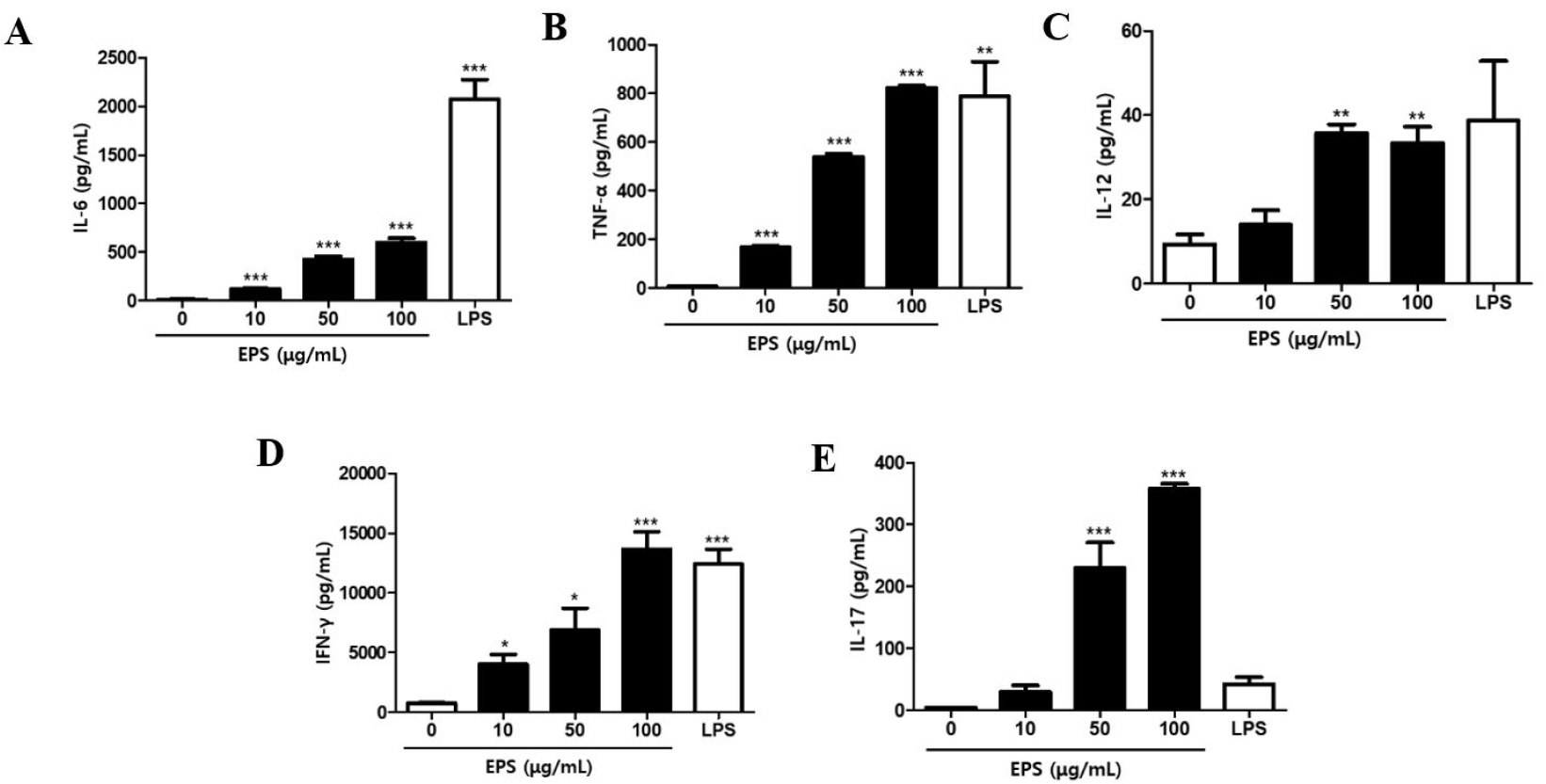 | Fig. 3Effect of EPS on cytokine secretion by splenocytes. Splenocytes were stimulated with EPS or LPS (200 ng/mL) for 24 h. The cell culture supernatants were harvested and used to analyze cytokines levels by ELISA kits. Data are expressed as the mean ± SEM of triplicate samples. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group. |
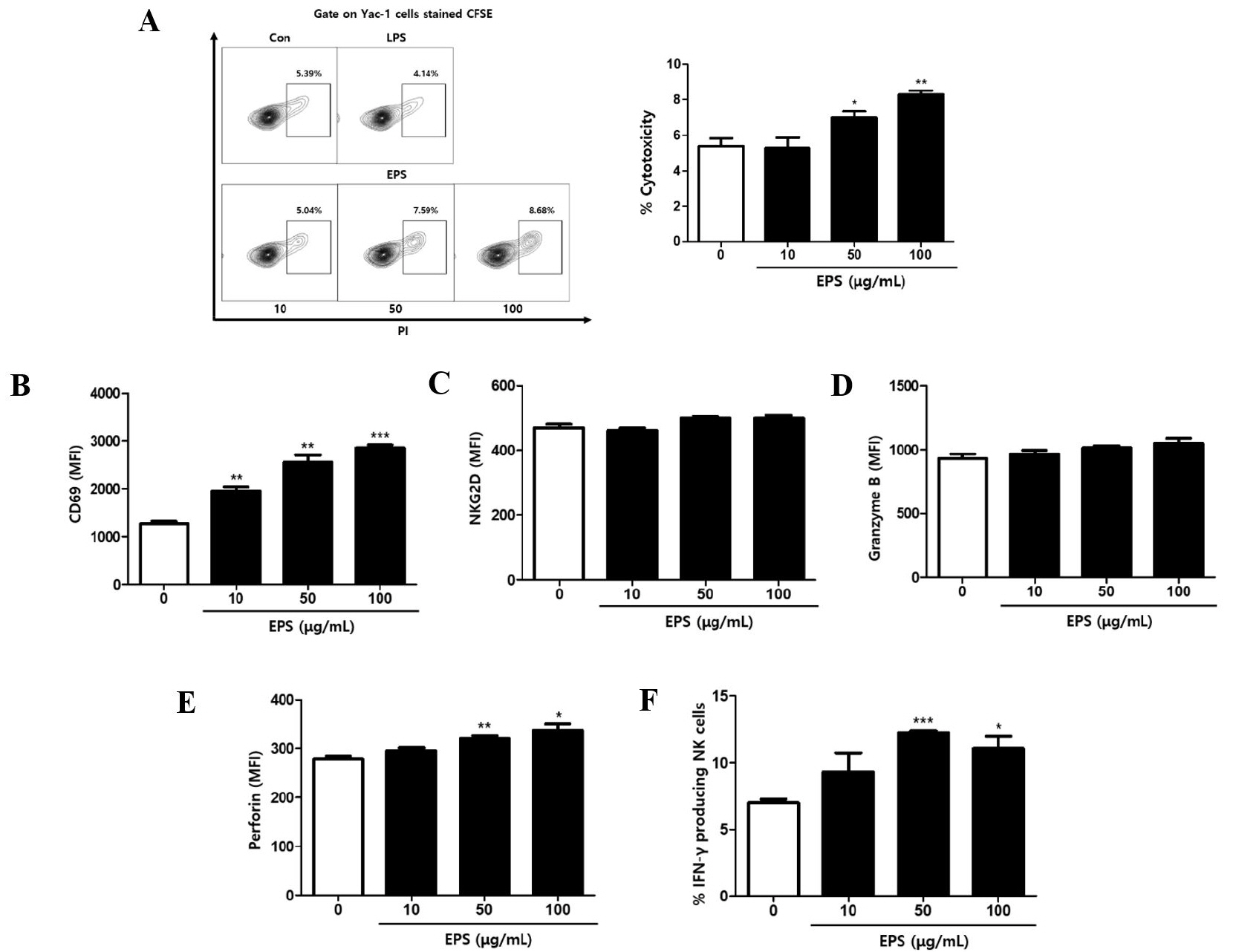 | Fig. 4Effect of EPS on NK cell activation. Splenocytes were treated with EPS at 10, 50, and 100 μg/mL. (A) After 22 h, these cells were co-cultured at a 5:1 ratio with CFSE-labeled Yac-1 cells for 2 h. Then, cells were stained with PI. The frequency of killed Yac-1cells (PI+ gated on CFSE) was detected by flow cytometry. (B–F) After incubation for 24 h, cells were stained with antibodies against CD3, CD49b, CD69, NKG2D, granzyme B, perforin, and IFN-γ and detected by flow cytometry. Cells were gated on CD3-CD49b+ NK cells, and MFI or percentage of each staining is shown. Data are expressed as the mean ± SEM of triplicate samples. *p < 0.05, **p < 0.01, ***p < 0.001 compared with control group. |
Oral administration of JW15 increases NK cell activity in mice
According to the in vitro experiment, it is plausible that the administration of live JW15 may enhance NK cell activity. To test the effect of JW15 in vivo, mice were orally administered either with live JW15 or with LGG daily, for 14 days. Then, mice splenocytes were isolated and tested for NK cell cytotoxicity against Yac-1 cells. Unlike the administration of LGG, JW15 administration significantly enhanced the level of killed target cells (Fig. 5A). Unexpectedly, the frequency of NK cells among splenocytes was increased by both LAB species (Fig. 5B). In addition, stimulation of splenocytes with IL-2 (20 ng/mL) for 18 h showed up-regulated IFN-γ secretion in JW15-treated mice compared to the control or LGG-treated group (Fig. 5C). However, the effect of JW15 on IFN-γ induction was not significant. Finally, our in vivo results suggest that oral administration of JW15 enhances NK cell activity in mice.
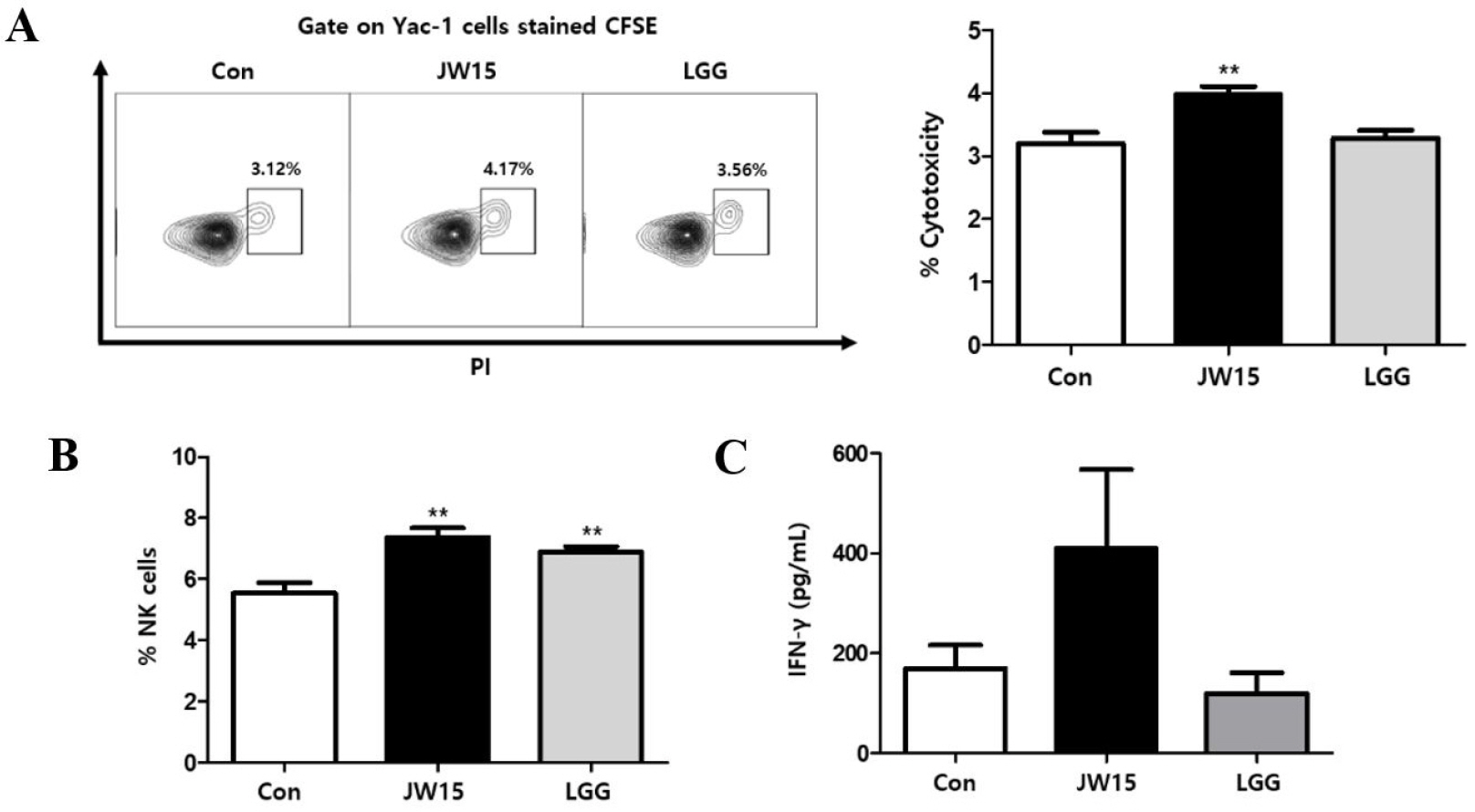 | Fig. 5Effect of oral administration of JW15 on NK cells. C57BL/6 mice were administered with JW15 or LGG daily for 14 days and then euthanized to harvest spleen. (A) A single-cell suspension of splenocytes was co-cultured with CFSE-labeled Yac-1 cells at a 10:1 ratio for 4 h. Then, cells were stained with PI and analyzed by flow cytometry for PI+ gated on CFSE-stained cells as killed Yac-1 cells. (B) Splenocytes were stained for NK cells (CD3-CD49b+) and subjected to flow cytometry analysis or (C) treated with IL-2 (20 ng/mL) for 18 h, and then secreted IFN-γ in the supernatants was detected by ELISA. Data are expressed as the mean ± SEM of triplicate samples. **p < 0.01 compared with control group. |
Go to : 
DISCUSSION
The beneficial effects of LAB on human health have been studied for many years. The immune system plays an essential role in most human diseases, including cancer, obesity, bacterial or viral infection, and cardiovascular diseases (43). Some LAB species are able to stimulate or suppress both innate and acquired immune responses (44). Therefore, immuno-modulatory functions of LAB have been considered as one of the most important aspects of LAB on human health. Recently, NK cell activation by LAB was demonstrated by several groups and suggested as a promising strategy to control viral infection and prevent cancer (45, 46). Here, we provide in vitro and in vivo evidence that W. cibaria JW15 (isolated from kimchi, a Korean traditional fermented food) effectively activates NK cells and enhances their cytotoxic activity against tumor cells.
NK cells are well-known as sentinel cells of the innate immunity system. They represent a major component of cytotoxic lymphocytes with the ability to recognize abnormal cells, such as tumor cells and virus-infected cells, and kill those cells without pre-sensitization (26). In addition, NK cells also play a role in adaptive immunity (47), such as the augmentation of T cell-mediated immune responses by interacting with dendritic cells. The diverse functions and essential roles of NK cells in protective immunity, especially against viral infection and cancer, have suggested that augmenting the NK cell response could be an attractive strategy to develop prophylactics or therapeutics for those diseases. We demonstrated that JW15 acted as an effective NK cell activator and increased the cytotoxic response of NK cells against a tumor cell line in vitro. It was found that JW15 directly activated NK cells and increased intracellular cytolytic molecules, such as perforin and granzyme B, as well as IFN-γ, a potent immunostimulatory cytokine. Yamane et al. demonstrated that a mixture of kefir-isolated LAB increased the tumor cell cytotoxicity of an NK cell line in vitro (48), providing evidence for the direct effect of LAB on NK cells. We further confirmed the stimulatory effect of JW15 on NK cells in vivo. Daily administration of live JW15 via oral gavage enhanced the cytotoxic effect of NK cells on cancer cells ex vivo, consistent with a previous study that showed daily consumption of JW15 enhanced NK cell activity in non-diabetic subjects (49). These findings imply that the consumption of live JW15 could help prevent tumorigenesis by increasing NK cell-mediated surveillance system. However, further animal disease model studies are necessary to confirm our finding, which was revealed in normal mice. Oral administration of LAB is reported to ameliorate cyclophosphamide-induced immunosuppression by activating NK cells in mice (50).The antitumoral effect of LAB treatment has also been studied in mouse model. For instance, preventive intranasal treatment of Lactobacillus casei BL23 delayed tumor onset through a mechanism partially associated with NK cell activation and recruitment (51). Similarly, in a mouse study, L. casei Shirota delayed tumorigenesis induced by 3-methylcholanthrene (52). NK cell activation by LAB oral administration was also suggested as an effective prophylactic regimen for viral infection. LGG, L. pentosus S-PT84, L. plantarum nF1, and L. delbrueckii OLL1073R-1 enhanced host immunity via NK cell activation and protected mice from influenza A virus infection (53, 54, 55, 56).
Many LAB species are known to produce EPS, glycan structures present outside of the bacterial cell wall. The beneficial properties of LAB-produced EPS have drawn considerable interest for their immune-modulatory effect. As mentioned above, some studies reported that the EPS produced by LAB regulates both innate and adaptive immunity, leading to health-promoting benefits (41). It is recognized that the ability of EPS to stimulate immune cells differs among LAB species or strains, possibly due to structural differences of EPS (42). Hence, the search for EPS-producing LAB strains, especially in high amounts, is of great interest. Weissella cibaria is well-known to produce copious amounts of EPS compared to other LAB (57). However, the immunostimulatory role of EPS produced by JW15 has not yet been fully studied. To address this knowledge gap, we sought to determine whether EPS produced by JW15 stimulates cytokine production in splenocytes and examine the cytotoxic activity of NK cells in vitro. The secretion of a significant amount of cytokines by splenocytes treated with EPS reflected the immune-stimulatory activity of EPS, and this was further confirmed by the increased NK cell activation markers and cytotoxicity against tumor cells. In accordance with the results of a study, EPS derived from yogurt fermented with L. delbrueckii OLL1073R-1 enhanced NK cell cytotoxicity in vitro (56). Moreover, EPS isolated from L. rhamnosus KL37 and RW-9595M was shown to induce pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-12, in mouse peritoneal macrophages and, RAW 264.7 and peripheral blood mononuclear cells, respectively (58). Results of our and other groups suggest that EPS from LAB possess immunostimulatory properties and lead to proposing EPS as a new bioactive molecule with potential nutraceutical and pharmaceutical applications. However, our findings were based upon a simple in vitro study. Further studies are necessary to decipher the potential role of EPS form JW15 in an animal model that involves complex interactions among immune cells and various other cells and with the intestinal microbiome.
The present study demonstrated that live JW15 activates NK cells, important immune cells in the innate immune response, by increasing the expression of various activation markers, such as CD69 and NKG2D, and components of cytolytic granules. Additionally, we showed that EPS from JW15 enhanced the activation of NK cells. Moreover, the daily administration of live JW15 up-regulated the frequencies and cytotoxic activity of NK cells. Taken together, these findings suggest that enhancing NK cell-mediated immune responses by the consumption of live JW15 serve as a prophylactic strategy to prevent tumorigenesis and viral infection. Further studies are necessary to confirm immunostimulatory effects of JW15 and EPS in animal models of viral infection or cancer and reveal the detailed immunological and molecular mechanisms utilized by JW15 and EPS isolated from JW15.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download