INTRODUCTION
The emergence of bacterial pathogens that are resistant to commonly used antimicrobials is one of the most serious threats to public health (1). For instance, carbapenem resistance in gram-negative bacteria raises therapeutic challenges because carbapenems have been considered the most effective antibiotics against infections caused by multi-drug resistant (MDR) or extensively drug-resistant (XDR) pathogens (2). To combat with these MDR or XDR pathogens, tigecycline is used as a last-line antibiotic (3). Bacterial isolates resistant to tigecycline have become more abundant in recent decades.
Understanding the mechanism of antibiotic action as well as the mechanism of antibiotic resistance is critical for optimizing the clinical efficacy of antibiotics while preventing the development of antibiotic resistance. Thus, it is important to study the effect of altering clinical conditions on antibiotic activity (4). Tigecycline binds to the bacterial 30S ribosome and blocks the entry of tRNA, preventing protein synthesis (5). It is known that tigecycline forms reversible complexes with divalent cations, especially Mg2+ (6). Complexation with divalent cations is important for the biological action of tigecycline.
In this study, we investigated the effects of divalent cations in an extracellular environment on the in vitro activity of tigecycline against four species of gram-negative bacteria. The results showed that divalent cations in the extracellular environment reduced the antibacterial efficacy of tigecycline in all bacterial species investigated.
MATERIALS AND METHODS
We investigated four gram-negative bacterial species: Klebsiella pneumoniae (K. pneumoniae), Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), and Acinetobacter baumannii (A. baumannii). For each bacterial species, two strains were examined (Table 1). In vitro antimicrobial susceptibility testing was performed on strains to determine minimum inhibitory concentrations (MICs) by the broth microdilution method, according to the Clinical and Laboratory Standards Institute guidelines (7). Tigecycline was obtained from Pfizer (Korea), and tetracycline was purchased from Sigma-Aldrich (St. Louis, MO, USA). E. coli ATCC25922 and P. aeruginosa ATCC27853 were used as control strains. MICs were determined using cation-adjusted Mueller-Hinton broth (MHBII) and MHBII with added Mg2+ or Ca2+. Magnesium sulfate heptahydrate (24.65 g) was dissolved in autoclaved distilled water (87 mL) to prepare 100 mL of 1 M magnesium sulfate stock solution. Calcium chloride dihydrate (14.70 g) was dissolved in autoclaved distilled water (94.5 mL) to prepare 100 mL of 1 M calcium chloride stock solution. Final concentrations of Mg2+ or Ca2+ in MHBII were adjusted to 2.9 mM or 3.5 mM, respectively, based on severe hypermagnesemia and hypercalcemia criteria levels (3, 5, 8, 9, 10).
Table 1.
Minimum inhibitory concentrations of each strain of four gram-negative bacteria in MHB II and MHB II with added divalent cations
Additionally, we examined the survivability of the bacterial strains by measuring bacterial growth after exposure to tigecycline. Bacterial strains were cultured in MHBII at 37°C overnight. Cultures were diluted (1/100) in MHBII alone and MHBII with added Mg2+ or Ca2+. The final concentrations of divalent ions in MHBII with added Mg2+ or Ca2+ were 2.9 mM and 3.5 mM, respectively. Tigecycline was added to the three media at the MICs for each strain. After incubation at 37°C for 24 hours, OD600 was measured using GeneQuant 1300 (Biochrom Ltd., Cambourne, UK); subsequently, bacterial survivability was compared among the three media. Independent experiments were performed in triplicate. To compare the two groups, Pearson χ² tests and Fisher’s exact tests were used for categorical variables and Student’s t-test and Mann–Whitney U tests were utilized for continuous variables, as appropriate. All statistical tests were two-tailed.
RESULTS AND DISCUSSION
All strains of three gram-negative bacterial species (K. pneumoniae, E. coli, and A. baumannii) were susceptible to tigecycline in MHB II media alone (MICs: 0.25 to 1 mg/L; Table 1). It is known that P. aeruginosa is intrinsically tigecycline-resistant (9). While the reference strain of P. aeruginosa (ATCC27853) was resistant to tigecycline, the P. aeruginosa clinical strain 08-B099 was susceptible. When Mg2+ or Ca2+ was added to the MHBII media, MICs increased 2- to 4-fold in all strains, including the tigecycline-resistant P. aeruginosa strain ATCC27853 (Table 1).
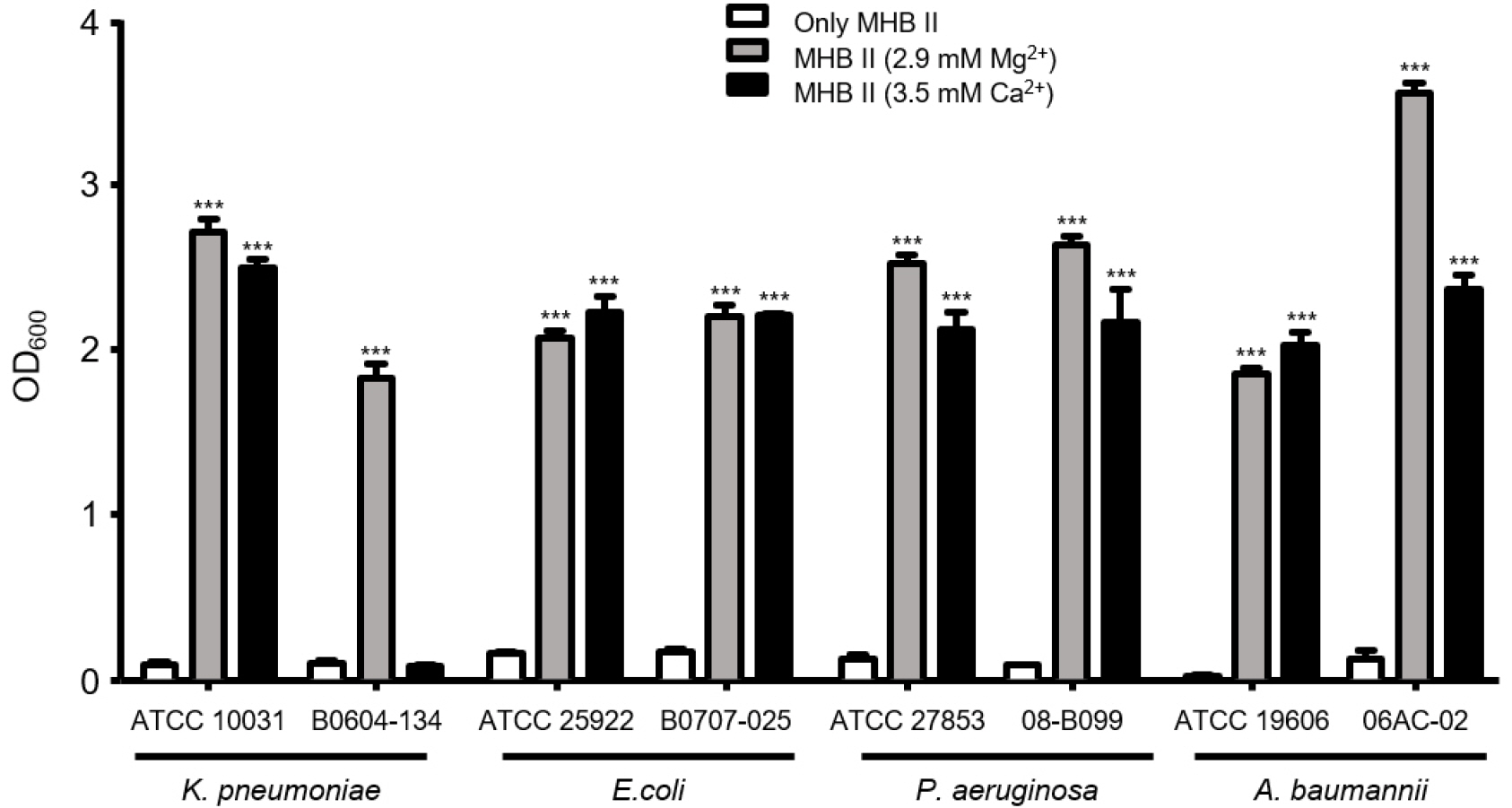
We compared the effect of Mg2+ or Ca2+ addition on the MIC using tetracycline, an antibiotic with a mechanism of action similar to that of tigecycline (Table 1). Tetracycline MICs increased in the MHBII media with added Mg2+ or Ca2+ for all strains. Survival rates were evaluated after exposure of bacterial strains to tigecycline for 24 hours. Bacterial strains were almost completely killed in the MHBII media with no additional cations by tigecycline at the MICs (Fig. 1). However, all strains, except K. pneumoniae clinical strain B0608-134, showed significantly higher survivability rates in the MHBII media with additional Mg2+ or Ca2+ (P<0.0001). While K. pneumoniae B0608-134 showed high survivability against tigecycline in MHBII with additional Mg2+, survivability did not increase when Ca2+ was added to the MHBII media.
Fig. 1
Comparison of antibiotic activity of tigecycline leading to divalent cations. Each bacteria strain was cultured in MHB II with or without divalent cations for 24 hours.
Complexation between tigecycline and divalent cations is necessary for the drug to cross the bacterial outer membrane through hydrophilic porins, such as OmpF and OmpC (11). Positively charged complexes transverse the porin following the Donnan potential caused by the distribution of ions across the outer membrane (12). In the periplasm, tigecycline dissociates from the divalent cation and becomes a protonated uncharged form, which then diffuses through the bacterial inner membrane into the bacterial cytoplasm. Thus, divalent ions are necessary for the antibiotic activity of tigecycline.
However, our data show that the concentration of divalent cations, such as Mg2+ and Ca2+, in the media influence the efficacy of tigecycline against gram-negative pathogens. In fact, high concentrations of these cations may inhibit the antibiotic activity of tigecycline. In our study, only high Ca2+ concentrations resulted in survival differences between the reference and clinical strains of K. pneumoniae. We tested additional clinical strains of K. pneumoniae and found similar results. Thus, the effect of Ca2+ on antibiotic efficacy may vary among bacterial species or strains.
Previous studies have shown that the type of medium can influence susceptibility of Enterobacteriaceae to tigecycline and tetracycline (8, 9, (10, 12, 13, 14). In addition, it was shown that the concentrations of Mg2+ and Ca2+ varied in the agar media used for the susceptibility tests (4). The results of these studies suggest that determination of tigecycline susceptibility should be performed with caution; moreover, fresh media should always be used during testing.
Similarly with our results, it was reported that a metal ion chelator such as EDTA could enhance the antibacterial effect of tigecycline, which was reversed by addition of divalent metal ions (15). However, in this study, we focused on the effect of levels of metal ions tantamount to criteria levels of hypermagnesemia and hypercalcemia in humans. In this regard, our study has difference with previous study. Moreover, we showed that metal ions could affect the tigecycline activity in A. baumannii and levels of calcium had less effect in K. pneumoniae.
The low activity of tigecycline in high divalent cation concentrations may have important implications with regard to the treatment of bacterial infections. Physiological levels of Mg2+ are critical for membrane and ribosome stabilization, neutralization of nucleic acids, and many enzymatic reactions (16). Ca2+ is also critical for maintaining physiological activity (17). As the renal system plays an important role in maintaining normal serum Mg2+ levels within a narrow range, kidney failure or end-stage renal disease causes hypermagnesemia due to a dysfunction in the process that maintains serum Mg2+ levels; it is defined as serum Mg2+ levels >1.1 mmol/L. The hypermagnesemia, which is often occurred in patients with acute bacterial infections such as bronchopneumonia and urinary tract infections, can lead to cardiovascular complications and neurological disorder (18). Our results suggest that tigecycline may be less effective in patients with high concentration of Mg2+ or Ca2+, resulted from urinary tract infections (18), regardless of the results of standard antibiotic susceptibility tests.
It is not clear why high concentrations of Mg2+ or Ca2+ lower the antibiotic efficacy of tigecycline. High levels of divalent cations forming complexes with tigecycline may delay antibiotic uptake into the bacterial cytosol. Otherwise, divalent cations may influence the function of tigecycline, inhibiting the ribosomal protein-synthesizing system. It is important to investigate why the concentrations of divalent cations lower the antibacterial activity of tigecycline. In addition, it is important that this is further evaluated in clinical studies.
In this study, we examined the effect of divalent cations on the in vitro activity of tigecycline against four species of gram-negative bacteria. Bacterial survivability increased significantly in media with high concentrations of Mg2+ or Ca2+. Our results suggest that caution should be exercised when using tigecycline to treat infected patients with high concentrations of Mg2+ or Ca2+.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download