INTRODUCTION
Colorectal cancer (CRC) is the most prevalent malignancy of the digestive tract, which is currently viewed as a serious health issue in the world (1). Overall, CRC is considered as the third most common cancer in males while in females it is ranked as the second most commonly occurring neoplasm (2). CRC is a multifactorial complex disease, which is influenced by a number of risk factors like the family history of CRC, diet, lifestyle and obesity, while inflammation is recognized as a crucial risk factor (3).
Human gut microbiota is comprised of approximately 1014 microorganisms which play a significant role in the maintenance of human health. These microbes perform a number of functions to regulate intestinal homeostasis, including host metabolism, mucosal barrier integrity and immunomodulation (4, 5). Several studies have confirmed that dysbiosis of microbiota is involved in the pathogenesis of various diseases specifically inflammatory bowel disease (IBD), neuropsychiatric disorders, cardiometabolic diseases and cancer (6).
Recent data showed a close relationship between particular bacterial species (Bacteroides fragilis, Escherichia coli, Streptococcus gallolyticus, Prevotella intermedia, Streptococcus bovis, Fusobacterium nucleatum and Enterococcus faecalis) and CRC (7, 8, 9, 10, 11, 12, 13, 14). Furthermore, it is evident from the previous report that the number of Fusobacterium nucleatum (F. nucleatum) is much higher in CRC patients as compared to healthy individuals (15). F. nucleatum promotes CRC through multiple mechanisms: including E-cadherin/β-catenin signaling (16), TLR4/MyD88 signaling (17), autophagy activation (18) and by suppressing host immunity (19) etc. (see the later section), while well-known anaerobic bacteria enterotoxigenic Bacteroides fragilis (ETBF) secretes Bacteroides fragilis-derived toxin (BFT) which can cause inflammation through Th17 cells (20, 21). Furthermore, BFT is involved in the upregulation of spermine oxidase (SPO) and generation of reactive oxygen species (ROS), resulting in DNA damage and promotion of intestinal tumorigenesis (22).
F. nucleatum is a gram-negative anaerobic bacterium that mostly presents in the oral cavity and intestine. Inside the oral cavity F. nucleatum can cause oral inflammation and is responsible for certain diseases like periodontitis and gingivitis (23, 24, 25), while it is also associated with brain abscesses, pancreatic cancer, liver abscesses and premature births (26, 27, 28, 29). F. nucleatum is the most prevalent bacterial species found in CRC tissues and its relevancy in CRC has been supported by a number of pre-clinical and clinical studies (9, 30, 31, (32). Previous studies have also confirmed that F. nucleatum associates with some anaerobic bacteria including Streptococcus, Leptotrichia and Campylobacter spp. which synergistically promotes the occurrence of CRC (33, 34). Metagenomic analysis using whole-genome sequencing (30), transcriptome sequencing (35) and bacterial 16S ribosomal RNA (rRNA) sequencing (36) have shown enrichment of F. nucleatum in CRC relative to adjacent normal tissues. In comparison to Bacteroidetes and Firmicutes, the levels of F. nucleatum shown to be high in CRC tissues (30, 31). Additionally, several studies have documented that the number of F. nucleatum increases in the tumor and fecal samples of CRC patients as compared to healthy cohorts (16, 32, 35, 37, 38, 39, 40). It was indicated that the presence of F. nucleatum in CRC is associated with the poor prognosis of disease and also promotes chemoresistance (18, 41). Actually, a high burden of F. nucleatum triggers a number of molecular events that are associated with CRC carcinogenesis, specifically microsatellite instability (MSI), CpG island methylator phenotype (CIMP) and tumorigenic mutations in TP53, BRAF, CHD7 and CHD8 genes (41, 42). The notion that F. nucleatum has a role in CRC tumorigenesis was first proved in Apcmin/+ mice (19), which was later supported by the findings that this bacterium promotes CRC by stimulating β-catenin pathway through FadA adhesin (43). Previous studies revealed that in comparison to F. nucleatum-negative cases, F. nucleatum-positive cases are associated with more advanced stages of CRC, proximal tumor location, MSI-high, CIMP-high and lower density of CD3+ T cells (9, 39, 44). Metagenomics analysis indicate that F. nucleatum is associated with human CRC, but whether this is an indirect or causal link remains unclear (45). F. nucleatum was previously regarded as a passenger bacterium in human intestinal tract (45, 46). Recently, it has been considered to be a potential initiator of CRC susceptibility (38, 42). It was further observed that F. nucleatum promotes colorectal tumorigenesis in Apcmin/+ mice (19). Together, these studies shed light on the fact that F. nucleatum plays an important role in the initiation, promotion and progression of tumor cell growth in CRC, supporting that F. nucleatum is the causal factor of CRC instead of a consequence.
Aiming to support the future research on F. nucleatum and to unveil new diagnostic and therapeutic possibilities for CRC, the present study will focus on the following topics in detail: methods used for the detection and quantification of F. nucleatum in CRC; pathogenesis of F. nucleatum in CRC; F. nucleatum promotes epigenetic changes in CRC; F. nucleatum suppresses host immunity in CRC; role of F. nucleatum in drug resistance; screening and prevention strategies of F. nucleatum-associated CRC; and clinical management of F. nucleatum in CRC patients.
Go to : 
DETECTION METHODS OF F. NUCLEATUM IN CRC
Previous studies have used various techniques for detecting F. nucleatum in CRC like such as quantitative real-time polymerase chain reaction (qPCR), fluorescent quantitative polymerase chain reaction (FQ-PCR), droplet digital polymerase chain reaction (ddPCR), enzyme-linked immunosorbent assay (ELISA) and fluorescence in situ hybridization (FISH) (47). In FQ-PCR method, Taqman (flurorescent) probe specific for target gene is used while in conventional qPCR, SYBR Green dye is used (48). Also, researchers detected F. nucleatum in different samples based on whole genome sequencing, CRC frozen tissues, feces, and formalin-fixed paraffin-embedded (FFPE) CRC tissues (47).
A study conducted on Chinese CRC patients used FQ-PCR and FISH methods to detect F. nucleatum, which revealed that 88 out of 101 (87.1%) cases had increased levels of F. nucleatum in frozen and FFPE tissues respectively (37). Two American studies measured F. nucleatum abundance in FFPE tissue by qPCR, in which 76/598 (13%) CRC patients exhibited positive response (39). Similarly, one Japanese research enrolled 511 CRC patients and detected F. nucleatum in 44 (8.6%) individuals from CRC FFPE tissues by the aid of qPCR (44). In addition to that, another Japanese study used qPCR to estimate the concentration of F. nucleatum from the DNA of patient’s CRC tissues, detected its presence in 111 out of 149 (74%) cases (42). One more Japanese research reported regarding F. nucleatum enrichment in CRC FFPE tissues of 286/511 (56%) patients by qPCR (16). Similar results were obtained in stool samples of Japanese CRC patients by ddPCR method, where 85/158 (54%) individuals had high levels of F. nucleatum (49). Also, researchers determined F. nucleatum by qPCR in FFPE tissues obtained from CRC patients and stated that it is abundant in colon cancers as compared to rectal cancers (50). Thus, the concentration of F. nucleatum steadily decreases from cecum to rectum.
In comparison to qPCR, ddPCR and FQ-PCR are regarded as more efficient and convenient methods of detecting low bacterial concentrations (51, 52). Scientists extracted DNA from frozen CRC and normal tissues and analyzed through whole-genome sequencing (30). It was revealed that CRC tissues were enriched with F. nucleatum as compared to normal tissues (30). Similar results were obtained in another study based on transcriptome sequencing, where F. nucleatum were present in abundance in CRC tissues as compared to adjacent normal tissues (35).
Go to : 
POTENTIAL ROLE OF F. NUCLEATUM IN CRC
The underlying pathogenic mechanisms of F. nucleatum in CRC have been studied by various researchers (Fig. 1) (53, 54). It has been demonstrated that three virulence factors of F. nucleatum play a critical role in the pathogenesis as well as metastasis of CRC. These factors are: fusobacterium adhesin A (FadA), lipopolysaccharides (LPS) and fusobacterium autotransporter protein 2 (Fap2), all of which are present on the outside of F. nucleatum (55, 56, 57). It is well known that the invasion of specific bacterium into the epithelial cells is the prerequisite step of F. nucleatum-positive CRC (58). FadA is a highly conserved virulence factor for F. nucleatum, that facilitates its attachment to both cancerous and non-cancerous cells (43). Interestingly, there are two forms of FadA : one is known as pre-FadA which anchors to the membrane and consists of 129 amino acids, while the other form is mature FadA which consists of 111 amino acids (59, 60). Additionally, when these two forms are mixed together then active FadA is formed, which has a potential role in the attachment and invasion into host epithelial cells (43). FadA binds to E-cadherin which is present in normal as well as CRC cells (16). Actually, E-cadherin has tumor suppressive properties, but FadA binding inhibits this function and stimulates the proliferation of CRC cells (61). E-cadherin is a glycoprotein, composed of an extracellular, transmembrane and a cytoplasmic domain. Cytoplasmic domain further binds to various cytosolic components, particularly β-catenin. FadA binds to E-cadherin, resulting in the phosphorylation and internalization of E-cadherin, that leads to its degradation. Loss of E-cadherin is not only associated with the reduction of cell-cell adhesive properties but also increases the cytoplasmic pool of β-catenin. Thus, β-catenin migrates into the nucleus and results in the activation of β-catenin-regulated transcription (CRT). This CRT promotes the genetic expressions of various genes such as oncogenes (c-myc, Ccnd1), wnt genes (wnt7a, wnt7b, wnt9a), genes encoding transcription factors (LEF-1, NF-κB, TCF1, TCF3, TCF4) and inflammatory cytokines (IL-6, IL-8, IL-18) (16). CRC tissues highly express D-galactose-β-(1-3)-N-acetyl-D-galactosamine (Gal-GalNac) polysaccharide to which another virulence factor Fap2 binds, that leads to enrichment of F. nucleatum in CRC tissues (62). Another research indicated that Fap2 is involved in the binding of F. nucleatum to T cell immunoreceptor with Ig and tyrosine-based inhibitory motif domains (TIGIT). Actually, TIGIT is an immune checkpoint receptor, which is present in NK and T cells. TIGIT has the potency to bind to multiple ligands (CD155 and CD112) present on antigen presenting and tumor cells as well as Fap2 (63). So, Fap2 binding can cause the phosphorylation of TIGIT receptor and inhibits the PI3k, MAPK and NF-κB signaling pathways (63). The inhibition of these pathways strongly reduces the cytotoxic activity associated with NK and T cells, and promotes tumor progression (64). Moreover, another study has manifested that F. nucleatum activates the TLR4/MyD88 signaling pathway by LPS, which results in the activation of NF-κB. NF-κB induces the expression of various proinflammatory genes that result in the production of inflammatory cytokines such as IL-1, IL-2, IL-6, IL-8 and IL-12. These cytokines create an inflammatory environment that promotes the progression of CRC (17). In summary, such studies based on F. nucleatum virulence factors provide new vision for the better understanding of F. nucleatum-associated CRC.
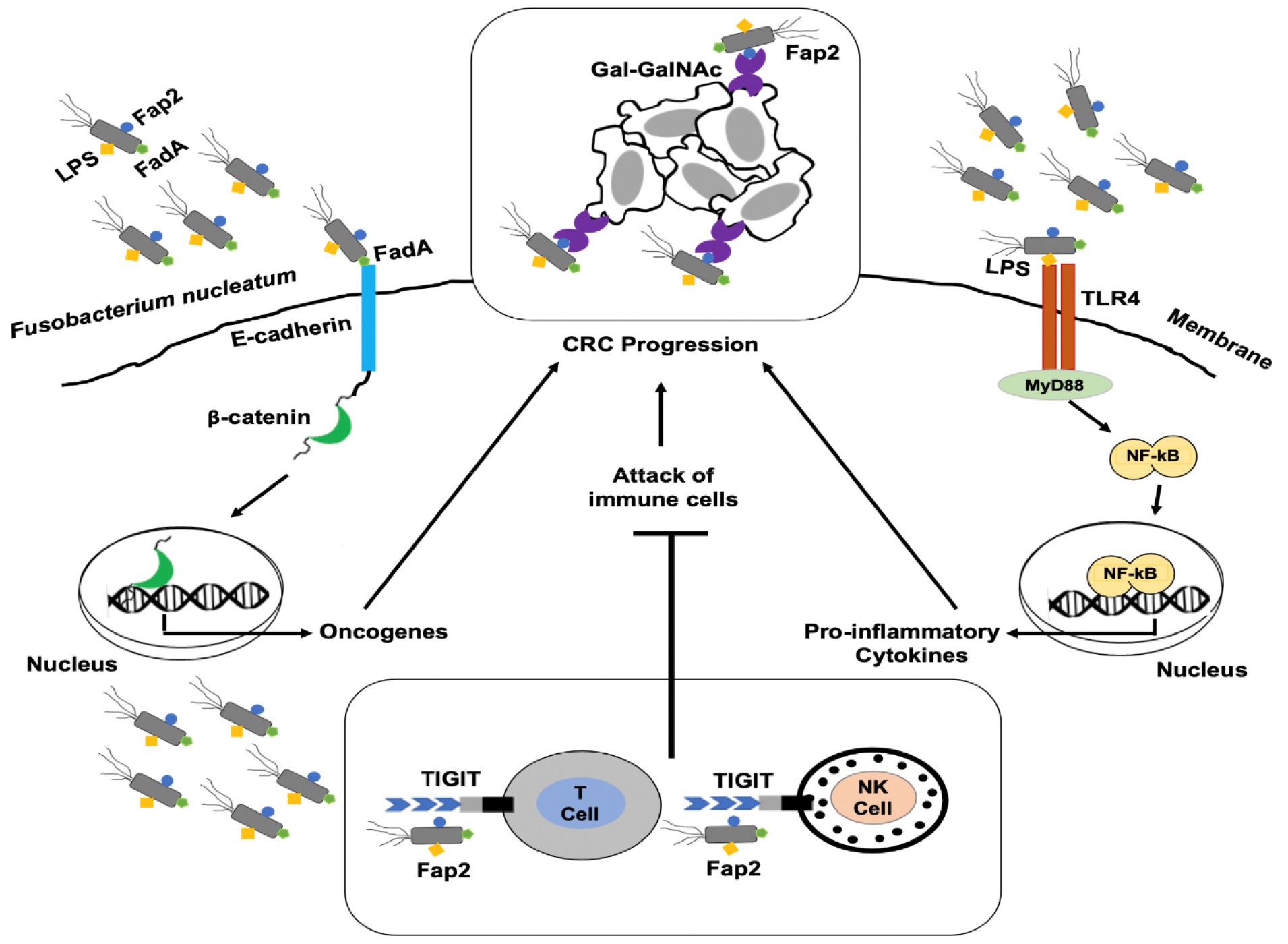 | Fig. 1Pathogenic mechanisms of F. nucleatum in CRC. F. nucleatum invades into the epithelial cells using virulence factors such as FadA, Fap2 and LPS. FadA attaches to the E-cadherin and activates the β-catenin signaling pathway, which promotes the expression of oncogenes. F. nucleatum recruits itself to the tumor sites by binding of Fap2 to Gal-GalNAc present on tumor cells. Additionally, Fap2 binds to the TIGIT receptor present on T and NK cells, that leads to inhibit their antitumor activity. Binding of LPS to TLR4 mediates TLR4/MyD88 signaling which leads to the translocation of NF-κB into the nucleus and results in the production of proinflammatory cytokines. Hence, all of these mechanisms potentiate the development and progression of CRC. CRC, colorectal cancer; FadA, fusobacterium adhesin A; Fap2, fusobacterium autotranporter protein 2; Gal-GalNAc, D-galactose-β-(1-3)-N-acetyl-D-galactosamine; LPS, lipopolysaccharides; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor-κB; NK, natural killer; TIGIT, T cell immunoreceptor with Ig and tyrosine-based inhibitory motif domains; TLR4, toll-like receptor 4. |
Go to : 
F. NUCLEATUM PROMOTES EPIGENETIC CHANGES IN CRC
It has been reported that F. nucleatum takes part in the epigenetic modifications of CRC. Previous findings have shown that F. nucleatum in CRC is associated with certain molecular features like high microsatellite index (MSI), CpG island methylator phenotype (CIMP) status, hypermethylation in the promoter region of mismatch repair protein (MLH1) and enhanced expression of miRNA-21 (42). Moreover, all these phenotypic changes mostly occur in the ascending colon of the gastrointestinal tract, which is also the major site for the residence of F. nucleatum (65, 66). These studies suggest that there is a close association between F. nucleatum and CRC microenvironment.
In case of CIMP, there is a hypermethylation of various CpG islands present in the promoter regions of several genes. Such increased methylation of the CpG island will lead to inactivation of tumor suppressor genes and promotion of chronic inflammation (67). MSI accounts for about 15-20% cases of CRC and is attributed to mutations in the DNA mismatch repair genes (42). F. nucleatum is responsible for increased production of reactive oxygen species and inflammatory cytokines, both of which contribute to the epigenetic silencing of the MLH1 and lead to MSI tumors (44).
miRNAs are recognized as small non-coding RNAs that function in RNA silencing as well as in the regulation of post-transcriptional gene expression. Also, miRNAs are now considered as diagnostic and therapeutic biomarker for a number of diseases, especially cancer (68). Previous data have proved that F. nucleatum might take part in increasing the levels of miRNA-21 through epigenetic regulation (32). Furthermore, miRNA-21 can increase the levels of IL-10 and prostaglandin E2 (PGE2), which suppress antitumor immunity mediated by T cells in the tumor microenvironment (69, 70). Therefore, high levels of miRNA-21 are usually associated with poor clinical outcomes (71). Further studies are required to clarify the underlying mechanisms responsible for F. nucleatum mediated epigenetic changes in the development of CRC.
Go to : 
F. NUCLEATUM AND HOST IMMUNITY
Several studies have proved that F. nucleatum assists CRC progression by regulating the tumor immune microenvironment (19, 72). Increased infiltration of CD11b+ myeloid cells was observed in Apcmin/+ mice that fed with F. nucleatum. Tumor-associated macrophages (TAMs), M2-like TAMS, dendritic cells, regulatory T cell (Treg) and T helper cell 17 (Th17) also increased in this mice model. Myeloid-derived suppressor cells (MDSC) subsets include monocytic and granulocytic cells which can suppress CD4+ T cells and activate NF-κB driven pro-inlammatory response. These results suggested that there is a link between F. nucleatum abundance and immunity in CRC microenvironment (19). F. nucleatum suppresses the host immune system through the following mechanisms:
TAMs
Many researchers have confirmed that the infiltration of TAMs increased in F. nucleatum-associated CRC (73). Moreover, F. nucleatum has a role in M2 polarization and promotes the progression of CRC through TLR4/STAT3 signaling pathway (74). F. nucleatum significantly produce butyric acid as the end product which can cause apoptosis of various immunoregulatory cells such as macrophages and lymphocytes (75). F. nucleatum is able to invade and survive in macrophages and creates the toxic microenvironment by promoting the expression of host indoleamine 2,3-dioxygenase (IDO). Additionally, this IDO disrupts the function of T lymphocytes and allows F. nucleatum-infected macrophages to escape from the attack of cytotoxic T lymphocytes (76).
T cells
The virulence factors of F. nucleatum have been known for their role in the inhibition of T cell activity (64, 77). It was observed that lower number of CD3+ and CD4+ T cells are present in F. nucleatum enriched CRC tissues (78). F. nucleatum is responsible for this reduction in T cell number by inhibiting proliferation while promoting apoptosis of T cells (79, 80, 81). For instance, F. nucleatum can do so through its inhibitory proteins that induce G1 phase arrest of the cell cycle (80). F. nucleatum promotes CRC by the release of short peptides along with short-chain fatty acids which subsequently cause the recruitment of various cells, particularly MDSCs. Then these MDSCs suppresses the function of CD4+ T helper cell and hence, further contributes in CRC tumorigenesis (82). Moreover, F. nucleatum virulence factors such as Fap2 and RadD can cause the death of human lymphocytes (77). In CRC, Fap2 can also inhibit the activity of T cells by directly binding to TIGIT, an immunoreceptor present on some T cells (64).
Other immune cells
All of the NK cells express TIGIT receptor which F. nucleatum binds to through Fap2. As a result of this binding, the killing function of NK cells is inhibited and promotes tumor progression (83). One of the studies has demonstrated that tumors from F. nucleatum-fed mice are rich in CD103+ dendritic cells as compared to the mice fed on a normal diet (19). Additionally, these dendritic cells can enhance the infiltration of Foxp3+ regulatory T cells which promote tumor growth by inhibiting the function of cytotoxic T cells (84). The infiltration of tumor associated neutrophils is more in F. nucleatum-fed mice in comparison to the control group (19). Recently it was indicated that the tumor associated neutrophils regulate antitumor immunity and have a role in the progression of CRC (72). These studies proposed that F. nucleatum is responsible for immune suppression and is associated with a poor prognosis of the disease (85).
Go to : 
ROLE OF F. NUCLEATUM IN DRUG RESISTANCE
Previous data shed light on the fact that F. nucleatum potentiates the development of tumor microenvironment. Drug resistance is a common problem which occurs during the CRC treatment, including either chemotherapy or targeted therapy (86, 87). Studies have shown that tumor microenvironment has an impact on drug resistance. Especially in case of CRC, a huge diversity of gut microbiota also has the potential to effect this tumor microenvironment (88). In this manner, F. nucleatum might play a role in promoting drug resistance by regulating tumor microenvironment of CRC. F. nucleatum potentiates drug resistance by the following mechanisms: first, it can mediate autophagy through TLR4 receptors. TLR4/MyD88 signaling pathway is activated by F. nucleatum, resulting in the inhibition of miRNA-18a and miRNA-4802. It was observed that miRNA-18a and miRNA-4802 negatively regulate specific autophagy related genes encoding such as Unc-51 like autophagy activating kinase (ULK1) and autophagy-related protein 7 (ATG7), respectively. Hence, the inhibition of these miRNAs by F. nucleatum promotes the induction of autophagy related proteins. Thus, increase in autophagy activity can inhibit the chemotherapy-induced apoptosis of CRC cells, that leads to chemoresistance (18). Second, F. nucleatum can enhance the chemoresistance of antiangiogenic agents such as bevacizumab. In CRC, the prominent role of F. nucleatum is to secrete proinflammatory cytokines (IL-6 and IL-8) that subsequently contribute in the pro-angiogenesis pathways (89). Third, F. nucleatum promotes the infiltration of myeloid derived suppressor cells, which are also known to contribute in chemoresistance (90, 91). F. nucleatum-mediated drug resistance can be reduced by the use of antimicrobial therapy.
Go to : 
SCREENING AND PREVENTION STRATEGIES OF F. NUCLEATUM-ASSOCIATED CRC
Identifying colorectal adenomas or early adenocarcinomas by screening can effectively reduce CRC mortality (92, 93). F. nucleatum can be used as a tool for diagnosis of CRC or for risk stratification in CRC screening (94). For screening of F. nucleatum-associated CRC, it is not feasible to obtain tissue samples while the more convenient way is to draw samples from serum and feces. It was mentioned in several studies that F. nucleatum infection leads to elevated levels of certain antibodies particularly anti-F. nucleatum (Fn)-IgA in the serum of CRC patients (95, 96). The combination of detection methods for anti-Fn-IgA with carcinoembryonic antigen and carbohydrate antigen 19-9 enhances the sensitivity to diagnose CRC in its early stages. These findings suggest that the marked increase in serum anti-Fn-IgA antibodies, act as potential marker for early detection of CRC cases (96). Moreover, another recent research used ELISA to measure the levels of antibodies against F. nucleatum in the serum samples of CRC patients and healthy individuals. It was observed that high levels of antibodies were present in F. nucleatum-positive CRC cases (95). Another non-invasive screening method includes fecal immunochemical test (FIT), but is not recommended for the detection of advanced adenomas due to less sensitivity (97). Later on it was observed that qPCR increase the sensitivity of FIT to 38.6 and 92.3% for advanced adenomas and CRC, respectively (40). One of the recent research has used qPCR to quantify F. nucleatum in stool samples, which manifested that levels of F. nucleatum in stool samples were significantly higher in the CRC group as compared with control and polyp group (98). Another study used ddPCR assay for detecting F. nucleatum in stool samples from Japanese population. It was concluded from results that in comparison to control group, F. nucleatum levels were significantly higher in the non-advanced adenoma group, advanced adenoma group and CRC group (49). Moreover, a loop-mediated isothermal amplification method has 10-fold more sensitivity as compared to qPCR which basically detect FadA virulence factor (99). So, in the future F. nucleatum can be used as a diagnostic marker for the early detection of CRC.
Numerous studies have been conducted to find the impact of diet on the prevalence of CRC (100, 101). It has been proved that diet high in grains, fruits and vegetables decrease the risk of CRC while a western-style diet rich in meat and fats increase its chances (102, 103, 104). High-fat-diet along with oncogene activation dampened paneth-cell mediated immunity and thus shift bacterial communities in such a way that promotes intestinal cancer (105). Although, the underlying reason for the contribution of diet in CRC prevalence is not clear, it has believed that gut flora might play an intermediate part in this association. Diet alters the human gut microbiome and long-term dietary patterns allow only specific microbes to retain in the gut while the others may become extinct (106, 107).
Previous studies focused on the reduction of F. nucleatum in CRC through diet modification (108, 109, 110). Diets rich in whole grains and dietary fiber are associated with a lower risk for F. nucleatum-positive colorectal cancer but not F. nucleatum-negative cancer, supporting a potential role for intestinal microbiota in mediating the association between diet and colorectal neoplasms (110). Diet may influence CRC risk by modulating F.nucleatum abundance as a dietary intervention study noted a marked increase in stool F.nucleatum levels after individuals were switched from a low-fiber to high-fat diet (109). Certain proinflammatory diets elevate the levels of IL-6, TNF receptor superfamily 1B (TNFRSF1B) and creatine protein (CRP), all of which contribute to systemic inflammation (101). Empirical Dietary Inflammatory Pattern (EDIP) score has been mostly used to determine the inflammatory effects of various diets. EDIP score has been assessed from eighteen foods that are known to elevate circulating levels of IL-6, TNFRSF1B and CRP (108). A previous study indicated that elevated EDIP scores are linked with high risk of F. nucleatum-associated CRC (100).
Go to : 
CLINICAL MANAGEMENT OF F. NUCLEATUM-ASSOCIATED CRC
F. nucleatum invades into the epithelial cells and release various cytokines which contribute to the development of tumor microenvironment and promote CRC progression (17, (111, 112, 113). As inflammation is one the causes of CRC occurrence, so anti-inflammatory agents have been considered to be good candidates for the prevention and treatment of CRC (114). Prostaglandin-Endoperoxide Synthase 2 (PTGS2) is an enzyme which is sufficiently expressed in several types of cancers, including CRC (115). Non-steroidal antiinflammatory drugs (NSAIDs) have been mostly prescribed to inhibit the activity of PTGS2 (116). Moreover, it was demonstrated that F. nucleatum enhances the production of this enzyme and promotes inflammatory cascade (111). Hence, it is possible to prevent the occurrence of CRC by the use of NSAIDs. In addition, sulindac, a NSAID, has been reported for the treatment of polyps in CRC patients (117). It was observed in a large-scale clinical trial that the continuous use of NSAIDs has a potential to reduce the risks associated with CRC (118). A low dose of aspirin can reduce the chances of polyp recurrence by 40-50% in CRC survivors (119, 120). Also, NSAIDs work as antitumor drugs by inhibiting Wnt/β-catenin pathway (121). In accordance with a prior study, prostaglandin E receptor 2 (PTGER2) enhances the levels of several proinflammatory cytokines by activating NF-κB pathway in cultured neutrophils. So, the expression of IL-6, TNF-α, CXCL1, COX-2 and Wnt5A increased in wild-type as compared to PTGER2 knockout mice (122). Hence, NSAIDs and PTGER2 antagonists may serve as potential preventive and therapeutic options against F. nucleatum-associated CRC.
Another option to treat F. nucleatum-associated CRC is by the use of antibiotics specific for this gram-negative bacillus. It was shown in CRC xenograft mouse model that metronidazole is effective in reducing both the F. nucleatum load as well as proliferation and growth of CRC tumors (123). These results suggest the use of particular antibiotics in CRC patients to reduce the burden of F. nucleatum. It is preferred to use the targeted narrow-spectrum antibiotics which do not disturb the normal gut flora for the treatment of F. nucleatum in CRC (124).
Immunotherapy is an emerging field to treat different types of tumors, based on various immune checkpoint inhibitors for cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death-ligand 1 (PD-L1) and programmed cell death 1 (PDCD1) (125). Complex colonic flora of CRC patients reduce the efficacy of PDCD1 inhibitors (126). Pembrolizumab, a PDCD1 inhibitor, was approved by the Food and Drug Authority (FDA) for the treatment of MSI-high solid tumors. As F. nucleatum-associated CRC is characterized by MSI-high, CIMP and BRAF mutations, hence PDCD1 inhibitors may be worthwhile for treating F. nucleatum-positive CRC cases (42, 127). Certainly, the use of PDCD1 inhibitors is limited due to a number of gastrointestinal side effects (128). Additionally, F. nucleatum uses its Fap2 virulence factor for binding with TIGIT receptor of T cells and NK cells and inhibits their antitumor function. In this respect, development of the anti-Fap2 antibody may play an efficient role in restoring the antitumor immune responses (64). Moreover, it was observed that the inhibition of miRNA-21 results in the reduction of metastasis of CRC cells (129). Recently, host-pathogen protein-protein interactions (HP-PPIs) clarified that there are 186 interactions between F. nucleatum and CRC-related host proteins that are contributed by 103 host proteins and 76 F. nucleatum proteins. Consequently, F. nucleatum-associated CRC can be treated by developing the drugs which can specifically target these HP-PPI (130).
Go to : 
CONCLUSION
F. nucleatum has been known to be involved in the pathogenesis of several human diseases including CRC. F. nucleatum is present in the abundance in CRC tissues and is linked with the poor prognosis of the disease. F. nucleatum invades into the mucosal epithelium through its virulence factors such as FadA and Fap2. Then F. nucleatum suppresses the host immune system through various molecular pathways and promotes progression of CRC. Chemoresistance is majorly observed in F. nucleatum-associated CRC, that results in reducing the efficacy of CRC chemotherapy. F. nucleatum can be used as a prognostic and therapeutic marker for CRC. Until now, there is no clinically available drug to treat F. nucleatum-associated CRC. Therefore, it is the need of the hour to design a targeted immunotherapy with minimal side effects for the efficient treatment of F. nucleatum-associated CRC.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download