1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323(13):1239–1242. PMID:
32091533.
2. Javanmardi F, Keshavarzi A, Akbari A, Emami A, Pirbonyeh N. Prevalence of underlying diseases in died cases of COVID-19: a systematic review and meta-analysis. PLoS One. 2020; 15(10):e0241265. PMID:
33095835.

3. Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021; 9(3):251–259. PMID:
33341155.

4. Reiner Benaim A, Sobel JA, Almog R, Lugassy S, Ben Shabbat T, Johnson A, et al. Comparing COVID-19 and influenza presentation and trajectory. Front Med (Lausanne). 2021; 8:656405. PMID:
34055833.

5. Modi C, Böhm V, Ferraro S, Stein G, Seljak U. Estimating COVID-19 mortality in Italy early in the COVID-19 pandemic. Nat Commun. 2021; 12(1):2729. PMID:
33980836.

6. O'Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DA, Azman AS, Paireau J, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021; 590(7844):140–145. PMID:
33137809.
7. Banerjee A, Pasea L, Harris S, Gonzalez-Izquierdo A, Torralbo A, Shallcross L, et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020; 395(10238):1715–1725. PMID:
32405103.

8. Mohamed MO, Gale CP, Kontopantelis E, Doran T, de Belder M, Asaria M, et al. Sex differences in mortality rates and underlying conditions for COVID-19 deaths in England and Wales. Mayo Clin Proc. 2020; 95(10):2110–2124. PMID:
33012342.

9. Omori R, Matsuyama R, Nakata Y. The age distribution of mortality from novel coronavirus disease (COVID-19) suggests no large difference of susceptibility by age. Sci Rep. 2020; 10(1):16642. PMID:
33024235.

10. Suh HJ, Lee E, Park SW. Clinical characteristics of COVID-19: risk factors for early oxygen requirement after hospitalization. J Korean Med Sci. 2021; 36(19):e139. PMID:
34002553.

11. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020; 323(16):1574–1581. PMID:
32250385.

12. Bennett KE, Mullooly M, O'Loughlin M, Fitzgerald M, O'Donnell J, O'Connor L, et al. Underlying conditions and risk of hospitalisation, ICU admission and mortality among those with COVID-19 in Ireland: a national surveillance study. Lancet Reg Health Eur. 2021; 5:100097. PMID:
33880459.

13. De Larochelambert Q, Marc A, Antero J, Le Bourg E, Toussaint JF. Covid-19 mortality: a matter of vulnerability among nations facing limited margins of adaptation. Front Public Health. 2020; 8:604339. PMID:
33330343.

14. Jang SY, Seon JY, Eun BL, Koh SB, Yoo JH, Lee WY, et al. Risk factors of outcomes of COVID-19 patients in Korea: focus on early symptoms. J Korean Med Sci. 2021; 36(18):e132. PMID:
33975399.

15. Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020; 18(1):206. PMID:
32434518.

16. Wu G, Yang P, Xie Y, Woodruff HC, Rao X, Guiot J, et al. Development of a clinical decision support system for severity risk prediction and triage of COVID-19 patients at hospital admission: an international multicentre study. Eur Respir J. 2020; 56(2):56.
17. Liu YP, Li GM, He J, Liu Y, Li M, Zhang R, et al. Combined use of the neutrophil-to-lymphocyte ratio and CRP to predict 7-day disease severity in 84 hospitalized patients with COVID-19 pneumonia: a retrospective cohort study. Ann Transl Med. 2020; 8(10):635. PMID:
32566572.

18. Yu Y, Wang X, Li M, Gu L, Xie Z, Gu W, et al. Nomogram to identify severe coronavirus disease 2019 (COVID-19) based on initial clinical and CT characteristics: a multi-center study. BMC Med Imaging. 2020; 20(1):111. PMID:
33008329.

19. Weng Z, Chen Q, Li S, Li H, Zhang Q, Lu S, et al. ANDC: an early warning score to predict mortality risk for patients with Coronavirus Disease 2019. J Transl Med. 2020; 18(1):328. PMID:
32867787.

20. Lee SW, Yang JM, Moon SY, Yoo IK, Ha EK, Kim SY, et al. Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. Lancet Psychiatry. 2020; 7(12):1025–1031. PMID:
32950066.

21. Yüce M, Filiztekin E, Özkaya KG. COVID-19 diagnosis - a review of current methods. Biosens Bioelectron. 2021; 172:112752. PMID:
33126180.
22. Du Y, Lv Y, Zha W, Zhou N, Hong X. Association of body mass index (BMI) with critical COVID-19 and in-hospital mortality: a dose-response meta-analysis. Metabolism. 2021; 117:154373. PMID:
32949592.

23. Heinze G, Dunkler D. Five myths about variable selection. Transpl Int. 2017; 30(1):6–10. PMID:
27896874.

24. Pan D, Cheng D, Cao Y, Hu C, Zou F, Yu W, et al. A predicting nomogram for mortality in patients with COVID-19. Front Public Health. 2020; 8:461. PMID:
32850612.

25. Zhou Y, He Y, Yang H, Yu H, Wang T, Chen Z, et al. Development and validation a nomogram for predicting the risk of severe COVID-19: a multi-center study in Sichuan, China. PLoS One. 2020; 15(5):e0233328. PMID:
32421703.
26. Yanez ND, Weiss NS, Romand JA, Treggiari MM. COVID-19 mortality risk for older men and women. BMC Public Health. 2020; 20(1):1742. PMID:
33213391.

27. Porcheddu R, Serra C, Kelvin D, Kelvin N, Rubino S. Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J Infect Dev Ctries. 2020; 14(2):125–128. PMID:
32146445.

28. Perez-Saez J, Lauer SA, Kaiser L, Regard S, Delaporte E, Guessous I, et al. Serology-informed estimates of SARS-CoV-2 infection fatality risk in Geneva, Switzerland. Lancet Infect Dis. 2021; 21(4):e69–70. PMID:
32679085.

29. Owen RK, Conroy SP, Taub N, Jones W, Bryden D, Pareek M, et al. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: a retrospective observational study using electronic health records. Age Ageing. 2021; 50(2):307–316. PMID:
32678866.

30. Chan JC, Tsui EL, Wong VC. Hospital Authority SARS Collaborative Group. Prognostication in severe acute respiratory syndrome: a retrospective time-course analysis of 1312 laboratory-confirmed patients in Hong Kong. Respirology. 2007; 12(4):531–542. PMID:
17587420.

31. Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013; 13(9):752–761. PMID:
23891402.

32. Liu C, Li L, Song K, Zhan ZY, Yao Y, Gong H, et al. A nomogram for predicting mortality in patients with COVID-19 and solid tumors: a multicenter retrospective cohort study. J Immunother Cancer. 2020; 8(2):8.

33. Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010; 47(3):193–199. PMID:
19333547.

34. Matias-Guiu JA, Pytel V, Matías-Guiu J. Death rate due to COVID-19 in Alzheimer's disease and frontotemporal dementia. J Alzheimers Dis. 2020; 78(2):537–541. PMID:
33074240.

35. Vrillon A, Mhanna E, Aveneau C, Lebozec M, Grosset L, Nankam D, et al. COVID-19 in adults with dementia: clinical features and risk factors of mortality-a clinical cohort study on 125 patients. Alzheimers Res Ther. 2021; 13(1):77. PMID:
33838684.

36. Ghaffari M, Ansari H, Beladimoghadam N, Aghamiri SH, Haghighi M, Nabavi M, et al. Neurological features and outcome in COVID-19: dementia can predict severe disease. J Neurovirol. 2021; 27(1):86–93. PMID:
33417193.

37. Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan L, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020; 71(15):833–840. PMID:
32296824.

38. Zeng F, Deng G, Cui Y, Zhang Y, Dai M, Chen L, et al. A predictive model for the severity of COVID-19 in elderly patients. Aging (Albany NY). 2020; 12(21):20982–20996. PMID:
33170150.

39. Cao G, Li P, Chen Y, Fang K, Chen B, Wang S, et al. A risk prediction model for evaluating the disease progression of COVID-19 pneumonia. Front Med (Lausanne). 2020; 7:556886. PMID:
33251226.

40. Acar HC, Can G, Karaali R, Börekçi Ş, Balkan II, Gemicioğlu B, et al. An easy-to-use nomogram for predicting in-hospital mortality risk in COVID-19: a retrospective cohort study in a university hospital. BMC Infect Dis. 2021; 21(1):148. PMID:
33546639.

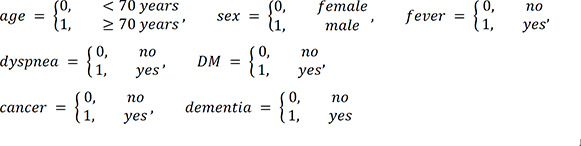




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download