This article has been
cited by other articles in ScienceCentral.
Abstract
There are still no agreed guidelines on the vaccination of coronavirus disease 2019 (COVID-19) for previously infected patients. Here, we present two seropositive healthcare workers (HCWs) working in an isolation ward who recovered from COVID-19 in April 2020 and got vaccinated with BNT162b2 vaccine in March 2021. We have assessed the clinical course, vaccine-related adverse events, and antibody response after natural infection and after first and second dose vaccination. One of the two HCWs was asymptomatic during quarantine, but the other had mild upper respiratory infection symptoms 1 day before admission, and the symptoms continued for 9 days. There was no pneumonic infiltration in chest X-ray in both patients, and no COVID-19 specific treatment was administered. Total immunoglobulin antibody and neutralizing antibody to anti-spike protein receptor-binding domain of severe acute respiratory syndrome coronavirus 2 were confirmed to be present in both HCWs in blood tests performed at 2 weeks and 4 weeks after discharge. Antibody response to mRNA vaccination showed marked elevation after the first vaccination, which was 30–40 times higher than that of antibody titer after natural infection in each patient (83.2 U/mL vs. > 2,500 U/mL in patient 1; 61.6 U/mL vs. > 2,500 U/mL in patient 2). Signal inhibition rate of neutralizing antibodies was also increased to over 97%. Due to this increased effect, there was little difference in antibody levels after the first and second dose. Both patients 1 and 2 suffered more from adverse vaccine reactions after the second vaccination than from COVID-19 symptoms.
Keywords: SARS-CoV-2, COVID-19 Vaccine, Neutralizing Antibodies, BNT162b2 Vaccine
INTRODUCTION
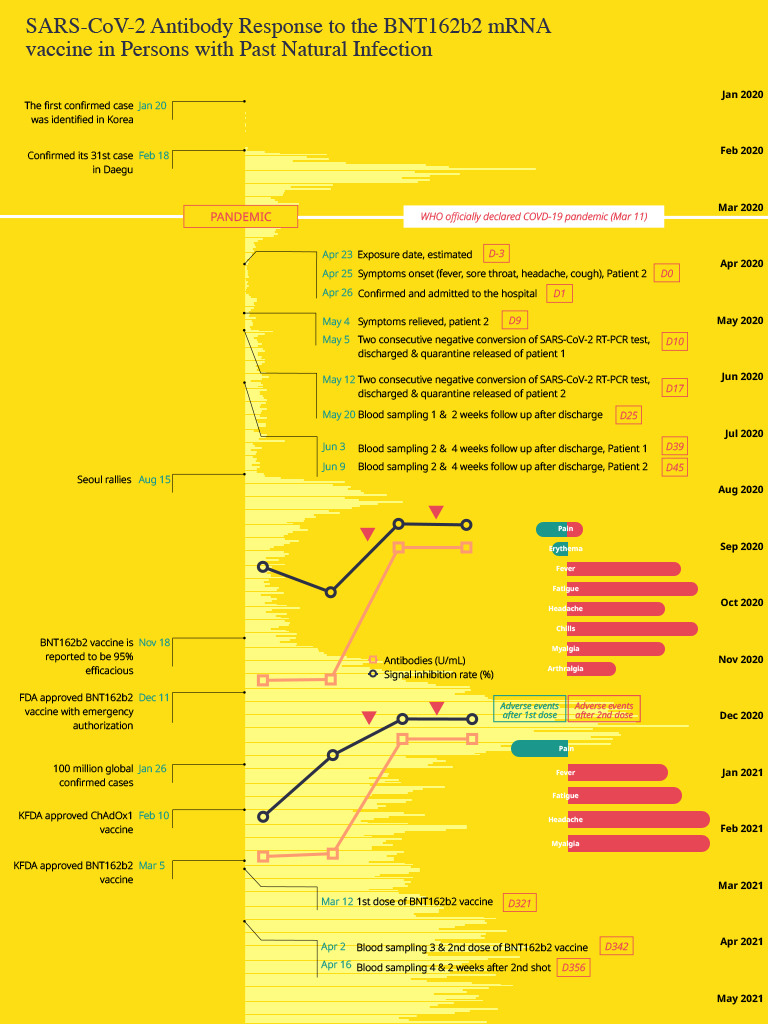
A worldwide vaccine campaign is underway to welcome the post-coronavirus disease 2019 (COVID-19) era. As of June 4, 2021, there have been 142,852 confirmed cases in Korea, and the number of vaccinated people who received at least one dose of COVID-19 vaccine is 7,086,292.
1 The number of confirmed cases worldwide is 173,020,740 and the vaccination rate varies greatly from country to country.
2 There is no clear guideline as to whether vaccination for previously infected patients is absolutely necessary. The Korea Disease Control and Prevention Agency recommends that people who have been previously infected should be vaccinated in the same way as the general public, because antibody formation is not confirmed in all infected people even when confirmed, and it is still unknown how long the established immunity lasts. In addition, if one has been treated for COVID-19 with monoclonal antibodies or convalescent plasma, it is recommended that you receive the same COVID-19 vaccination as non-infected persons after 90 days have passed.
3
However, there are still no agreed guidelines on when a person who has been infected should be vaccinated after recovering from COVID-19, or the appropriate dose of the vaccine. We report the cases of two healthcare workers (HCWs; patient 1, 27-year-old woman; patient 2, 38-year-old woman) who were confirmed to have COVID-19 and got vaccinated about one year later with the BNT162b2 mRNA vaccine; we measured the antibody titers after natural infection and after vaccination.
CASE DESCRIPTION
We describe the clinical course of two HCWs who, while working in a COVID-19 isolation ward in April 2020, were confirmed to have COVID-19 infection. We report changes in antibody titers after natural infection and after vaccination, as well as type and intensity of adverse events (AEs). One of the two HCWs was asymptomatic during the quarantine, but the other had mild URI symptoms like fever, headache, and sore throat for 9 days. No abnormal findings were found in routine blood tests, and no pneumonia was detected on chest X-ray. Oxygen therapy, antiviral drugs and steroids were not required. The clinical course and cycle threshold values of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) real-time polymerase chain reaction test during hospitalization are presented in
Fig. 1. Both HCWs returned to their duties after discharge and continued work in the COVID-19 isolation ward. About 10 months later, the BNT162b2 vaccine was supplied for quarantined hospital staff, and they were vaccinated with two doses at 3-week intervals (March 12, 2021, April 2, 2021). Longitudinal blood specimens of the two HCWs were taken from two different preexisting prospective studies. Blood samples were obtained at 2 and 4 weeks after discharge. They were compared with blood samples obtained at 3 and 2 weeks after the 1st and 2nd doses of BNT162b2 vaccinations, respectively. Antibody testing was performed using two methods: Elecsys
® Anti-SARS-CoV-2 S (Roche Diagnostics International Ltd, Rotkreuz, Switzerland) to quantitively detect antibodies (including IgG) against SARS-CoV-2 and cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit (GenScript, Piscataway, NJ, USA) to measure circulating neutralizing antibodies against the SARS-CoV-2. Both assays were performed according to the manufacturer' guideline. The antibody levels were interpreted as positive or negative with a 0.8 U/mL cutoff. And neutralizing antibody levels were interpreted by the signal inhibition rate (%) and it was classified into positive and negative with a 30% cutoff.
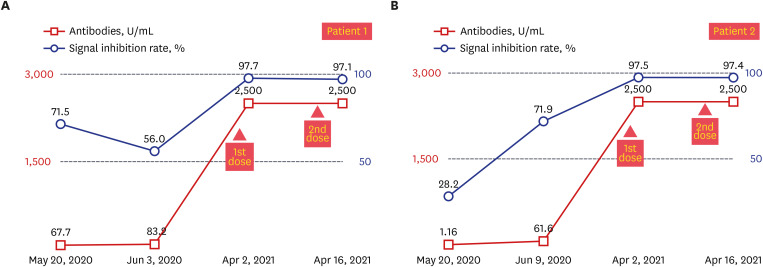
4 Although both cases had a mild infection and were asymptomatic, total immunoglobulin antibody and neutralizing antibody to SARS-CoV-2 were confirmed on days Day 25 (May 20, 2020) and Day 39 (Jun 3, 2020), respectively, from the onset of symptoms, which was not high. The change in antibody titer after vaccination is shown in
Fig. 2. In both cases, the total immunoglobulin antibody was 30–40 times higher than that of antibody titer after natural infection, reaching over 2,500 U/mL after the first dose of COVID-19 vaccination (83.2 U/mL vs. > 2,500 U/mL in patient 1; 61.6 U/mL vs. > 2,500 U/mL in patient 2), and the signal inhibition rates were 97.7% and 97.5%. There was no difference in antibody titers and signal inhibition rate between the first and second dose.
Fig. 1
Clinical course of two patients. (A) Clinical course of patient 1. (B) Clinical course of patient 2.
NPS = nasopharyngeal swab, UD = undetected.
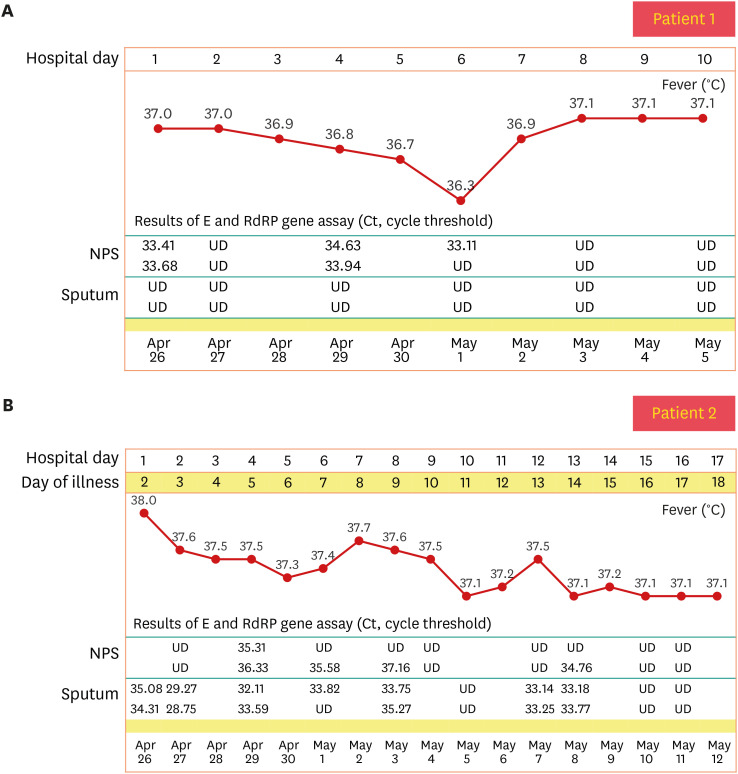
Fig. 2
Antibody response baseline, 1st, 2nd dose of the Pfizer vaccination in patients 1 and 2. Blue line shows a signal inhibition rate and orange line shows total immunoglobulins to severe acute respiratory syndrome coronavirus 2. (A) Antibody response and signal inhibition rate, patient 1. (B) Antibody response and signal inhibition rate, patient 2.
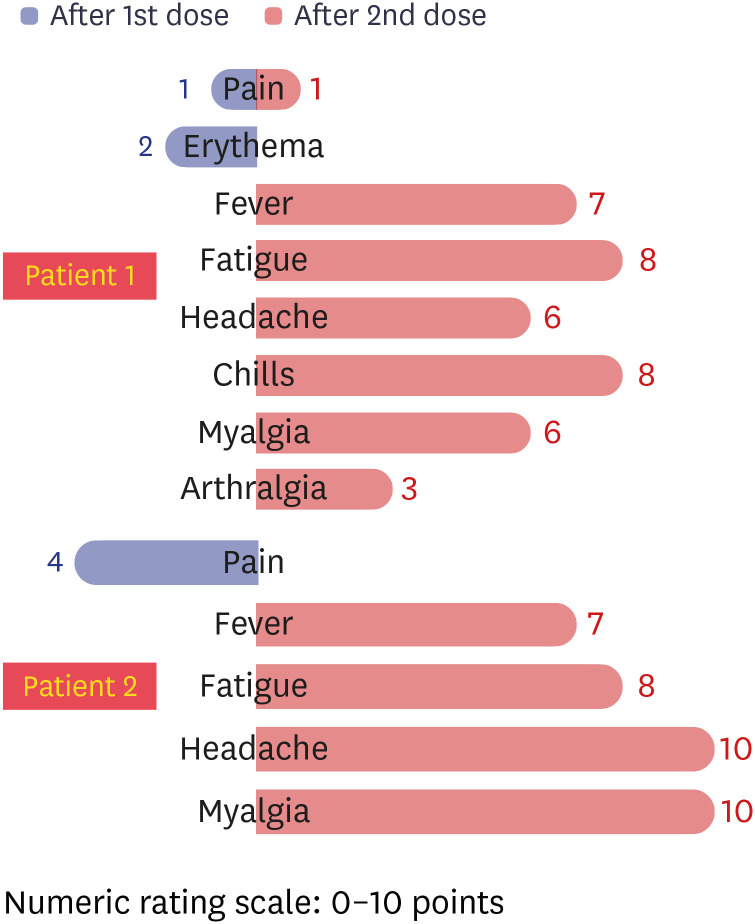
AEs after vaccination were evaluated by the investigator on an 11-point visual analog scale and toxicity scale, respectively (
Fig. 3).
5 Patient 1 had grade 1 injection site pain and erythema (2 points) for 3 days after the first dose, and her symptoms improved spontaneously without taking antipyretics. Patient 2 experienced grade 2 injection site pain (4 points) after the first dose, but improved within a day. There was no allergic reaction such as urticaria, facial or lip edema, in either of the cases, but more severe local and systemic AEs were experienced after the second dose than after the first dose. In patient 1, moderate fever (38.5°C), fatigue (8 points), headache (6 points), chills (8 points), myalgia (6 points), and arthralgia (3 points) persisted for 3 days, and gradually improved after administration of anti-pyretics (acetaminophen) for 3 days. After the second injection, patient 2 complained of moderate-intensity AEs, such as fever (38.5°C), fatigue (8 points), headache (10 points), and myalgia (10 points), and these symptoms persisted for 3–4 days. Patient 1 had no symptoms due to COVID-19 infection at first, but both HCWs suffered more from robust vaccine AEs related to the second vaccination than from symptoms due to COVID-19.
Fig. 3
Intensity of AEs after 1st and 2nd dose vaccinations of each patient. The intensities of AEs after 1st dose (blue bars, from midline to left side) and 2nd dose (red bars, from midline to right side) vaccinations are shown according to the visual analog scale.
AE = adverse event.
Ethics statement
The study was approved by the Institutional Review Board (IRB) of Myongji Hospital (IRB No. MJH2021-04-020, MJH2021-05-012, MJH2020-01-027). Blood samples were obtained with informed consent (MJH2020-01-027).
DISCUSSION
In this study, we report two HCWs who contracted natural SARS-CoV-2 infection and underwent a mild course of the disease; both developed antibodies after 6 weeks. Ten months later, the BNT162b2 mRNA vaccine was administered to them, and in both patients, the maximum level of antibody was developed with just one vaccination. Since this marked increase in antibody level after the first dose had already exceeded a measurable level, there was little difference in antibody levels between the first and second doses. Both patients reported that they suffered more from vaccine reactogenicity after the second dose than from the sickness due to natural infection.
In general, it is considered that the acquisition of immunity from natural infection is superior to vaccine-induced immunity. A previous study reported that 69.3% of the 143 previous infected COVID-19 patients showed sufficient neutralizing antibodies titer by natural immunity in the blood sample obtained on the 109th day from symptom onset.
6 In addition, according to a study of 52 patients who had mild COVID-19 infection, the SARS-CoV-2 antibodies were detected up to 1 year after the infection.
7 However, some reports have suggested that the immune response from mRNA COVID-19 vaccines is more robust than natural infection.
8 COVID-19 vaccine-induced antibody profile differs from the antibody profile induced by natural infection. When a natural infection with coronavirus occurs, antibodies can not only bind to the spike protein, but other antibodies to various other viral proteins such as nucleocapsid protein can be produced.
8 Since the measurements in this study did not include the entire antibody profile or level of cellular immunity acquired after prior infection, we measured only the SARS-CoV-2 receptor-binding domain antibody. Therefore, it is not possible to explain the full host immunity to SARS-CoV-2 infection by comparing absolute specific antibody levels.
In our study, total immunoglobulin antibody levels and neutralizing antibodies were significantly increased after vaccination compared to antibody levels at 4 weeks after natural infection (
Fig. 2). We did not know the antibody titer just before vaccination, but the antibody titer after the first vaccination exceeded the measurable threshold. These similar results show that the level of antibody titers after the first vaccine dose in two seropositive patients with natural infection elicited an effect similar to that of full vaccination in naïve patients. These findings were consistent with previous reports of high post-vaccine antibody titers in seropositive individuals.
91011 According to other reports, all but two of the 36 seropositive participants demonstrated boosting of antibody levels after the first dose, and no additional boosting effect was confirmed by the second dose. In the case of those vaccinated with no previous history of infection, the antibody titer after the first vaccination was similar to the antibody titer before vaccination of those who were previously confirmed.
12 According to a large-scale study of vaccine efficacy in Israel, the protection due to prior SARS-CoV-2 infection was similar in extent to the protection provided by the Pfizer BNT162b2 vaccine. Therefore, the need to vaccinate previously infected individuals was questioned.
13
In our case, high antibody titers were already confirmed after the first vaccination in individuals with confirmed past natural infection, and after the second vaccination, only the adverse reactions to the vaccine increased significantly without additional boosting effect. Wouldn't one booster be enough for a confirmed patient with antibodies? When comparing vaccination rates by country, there is still a large gap in vaccination between developed and less developed countries.
2 If the recommendation is changed based on the study results of the antibody against the previous COVID-19 infected person, it will save the vaccine for a single dose, improve the situation with regard to insufficient vaccine supply, and at the individual level, local and systemic AEs due to unnecessary secondary vaccination can have the effect of making the experience less painful. Given that there are already 130,000 COVID-19 survivors in Korea, further well-designed studies are needed to determine whether they need to be vaccinated or if they should be vaccinated equally along with the naïve population.
ACKNOWLEDGMENTS
We are grateful to the two healthcare workers (HCWs) of the Myongji Hospital for their dedicated effort to cope with the coronavirus disease 2019 (COVID-19) pandemic and for their participation in the study.
References
1. Korea Disease Control and Prevention Agency. Current COVID-19 vaccination status in Korea. Updated 2021. Assessed June 4, 2021.
https://ncv.kdca.go.kr/.
3. Centers for Disease Control and Prevention (US). Current COVID-19 vaccination dashboard. Updated 2021. Assessed June 4, 2021.
https://korean.cdc.gov.
4. Taylor SC, Hurst B, Charlton CL, Bailey A, Kanji JN, McCarthy MK, et al. A new SARS-CoV-2 dual-purpose serology test: highly accurate infection tracing and neutralizing antibody response detection. J Clin Microbiol. 2021; 59(4):e02438-20. PMID:
33500361.

6. Kim YJ, Bae JY, Bae S, Hwang S, Kwon KT, Chang HH, et al. Neutralizing antibody responses to SARS-CoV-2 in Korean patients who have recovered from COVID-19. Yonsei Med J. 2021; 62(7):584–592. PMID:
34164955.

7. Choe PG, Kim KH, Kang CK, Suh HJ, Kang E, Lee SY, et al. Antibody responses one year after mild SARS-CoV-2 infection. J Korean Med Sci. 2021; 36(21):e157. PMID:
34060263.

8. Assis R, Jain A, Nakajima R, Jasinskas A, Kahn S, Palma A, et al. Substantial differences in SARS-CoV-2 antibody responses elicited by natural infection and mRNA vaccination. bioRxiv. Forthcoming. 2021; DOI:
10.1101/2021.04.15.440089.

9. Krammer F, Srivastava K; the PARIS team, Simon V. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. medRxiv. Forthcoming. 2021; DOI:
10.1101/2021.01.29.21250653.

10. Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021; 397(10279):1057–1058. PMID:
33640038.

11. Bradley T, Grundberg E, Selvarangan R, LeMaster C, Fraley E, Banerjee D, et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021; 384(20):1959–1961. PMID:
33755375.

12. Tré-Hardy M, Cupaiolo R, Papleux E, Wilmet A, Horeanga A, Antoine-Moussiaux T, et al. Reactogenicity, safety and antibody response, after one and two doses of mRNA-1273 in seronegative and seropositive healthcare workers. J Infect. 2021; 83(2):237–279.

13. Goldberg Y, Mandel M, Woodbridge Y, Fluss R, Novikov I, Yaari R, et al. Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection: a three-month nationwide experience from Israel. medRxiv. Forthcoming. 2021; DOI:
10.1101/2021.04.20.21255670.







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download