INTRODUCTION
MATERIALS AND METHODS
Study design
Study outcomes
Statistical analysis
RESULTS
Surveillance study in RA
Table 1
| Characteristic | RA study (n=740) | LN study (n=307) | MG study (n=104) |
|---|---|---|---|
| Age (yr) | |||
| Mean (SD) | 53.7 (13.4) | 37.3 (11.9) | 53.5 (13.7) |
| Range | 7.0∼89.0 | 13.0∼39.0 | 21.0∼86.0 |
| Age group (yr) | |||
| <20 | 1 (0.1) | 15 (4.9) | 0 |
| 20 to <40 | 117 (15.8) | 169 (55.1) | 15 (14.4)* |
| 40 to <60 | 350 (47.3) | 110 (35.8) | 54 (51.9) |
| ≥60 | 272 (36.8) | 13 (4.2) | 35 (33.7) |
| Sex, female | 609 (82.3) | 273 (88.9) | 60 (57.7) |
| Duration of RA, LN, or MG, months | |||
| Mean (SD) | 74.2 (73.3) | 75.8 (64.9) | 92.0 (99.6) |
| Median | NA | 60.0 | 42.7 |
| Range | 1∼523 | 1∼281 | 0.48∼432 |
| Concomitant medications | 738 (99.7) | 307 (100.0) | 101 (97.1) |
| Current comorbidity | 409 (55.3) | 307 (100.0) | 82 (78.9) |
| Previous illness | NR | 90 (29.3) | 28 (26.9) |
| Current illness | NR | 307 (100.0) | 82 (78.9) |
| Hepatic impairment | 30 (4.1) | 11 (3.6) | 2 (1.9) |
| Renal impairment | 10 (1.4) | 108 (35.2) | 0 |
Table 2
| Event | No. patients or events (%) | |
|---|---|---|
| AEs | ADRs | |
| Patients with AE or ADR (n=740 patients) | 94 (12.7) | 62 (8.4) |
| AEs potentially related to tacrolimus* (n=123 AEs) | 79 (64.2) | NR |
| AEs leading to discontinuation of tacrolimus† (n=123 AEs) | 60 (48.8) | NR |
| Severity (n=123 AEs) | ||
| Mild | 76 (61.8) | NR |
| Moderate | 40 (32.5) | NR |
| Severe | 7 (5.7) | NR |
| AE or ADR occurring in ≥0.5% of patients (n=740 patients) | ||
| Gastrointestinal disorders | ||
| Abdominal pain | 8 (1.1) | 7 (1.0) |
| Dyspepsia | 5 (0.7) | 5 (0.7) |
| Nausea | 5 (0.7) | 5 (0.7) |
| Diarrhea | 4 (0.5) | 4 (0.5) |
| General disorders and administration site conditions | ||
| ESR increased | 6 (0.8) | 2 (0.3) |
| Hepatobiliary disorders | ||
| SGPT increased | 4 (0.5) | 3 (0.4) |
| Investigations | ||
| CRP increased | 5 (0.7) | 3 (0.4) |
| Renal and urinary disorders | ||
| Face edema | 4 (0.5) | 2 (0.3) |
| Skin and subcutaneous tissue disorders | ||
| Rash | 4 (0.5) | 4 (0.5) |
| SAEs (n=740 patients) | 5 (0.7) | 1 (0.1) |
| Cardiac disorders | ||
| Myocardial infarction | 1 (0.1) | 0 |
| Gastrointestinal disorders | ||
| Gastric ulcer | 1 (0.1) | 0 |
| General disorders and administration site conditions | ||
| Deterioration in physical condition | 1 (0.1) | 0 |
| Asthenia | 1 (0.1) | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Worsening RA | 1 (0.1) | 1 (0.1) |
| Neoplasms | ||
| Cervical carcinoma | 1 (0.1) | 0 |
| Skin and subcutaneous tissue disorders | ||
| Pruritus | 1 (0.1) | 0 |
| Surgical and medical procedures | ||
| Surgical intervention | 1 (0.1) | 0 |
ADR: adverse drug reaction, AE: adverse event, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, NR: not reported, RA: rheumatoid arthritis, SAE: serious adverse event, SGPT: serum glutamic-pyruvic transaminase. *Includes AEs classified as certainly, probably/likely, or possibly related to tacrolimus, or those recorded as conditional/unclassified or not assessable/unclassifiable. †AEs leading to discontinuation were abdominal pain, indigestion, diarrhea, nausea, constipation, unspecified gastrointestinal disorder, gastroenterocolitis, xerostomia, vomiting, gastric ulcer, elevated erythrocyte sedimentation rate, peripheral edema, asthenia, edema, deterioration in physical condition, pharyngitis, cough, hepatosis, cholelithiasis, elevated C-reactive protein, dizziness, hypoesthesia, headache, muscle pain, infectious arthritis, arthralgia, rash, itchiness, dermatitis, facial edema, panhematopenia, palpitation, high pulse rate, loss of appetite, hyperglycemia, dazzled vision, vaginitis, vaginal bleeding, and residual urine. 123 AEs were reported in 94 patients.
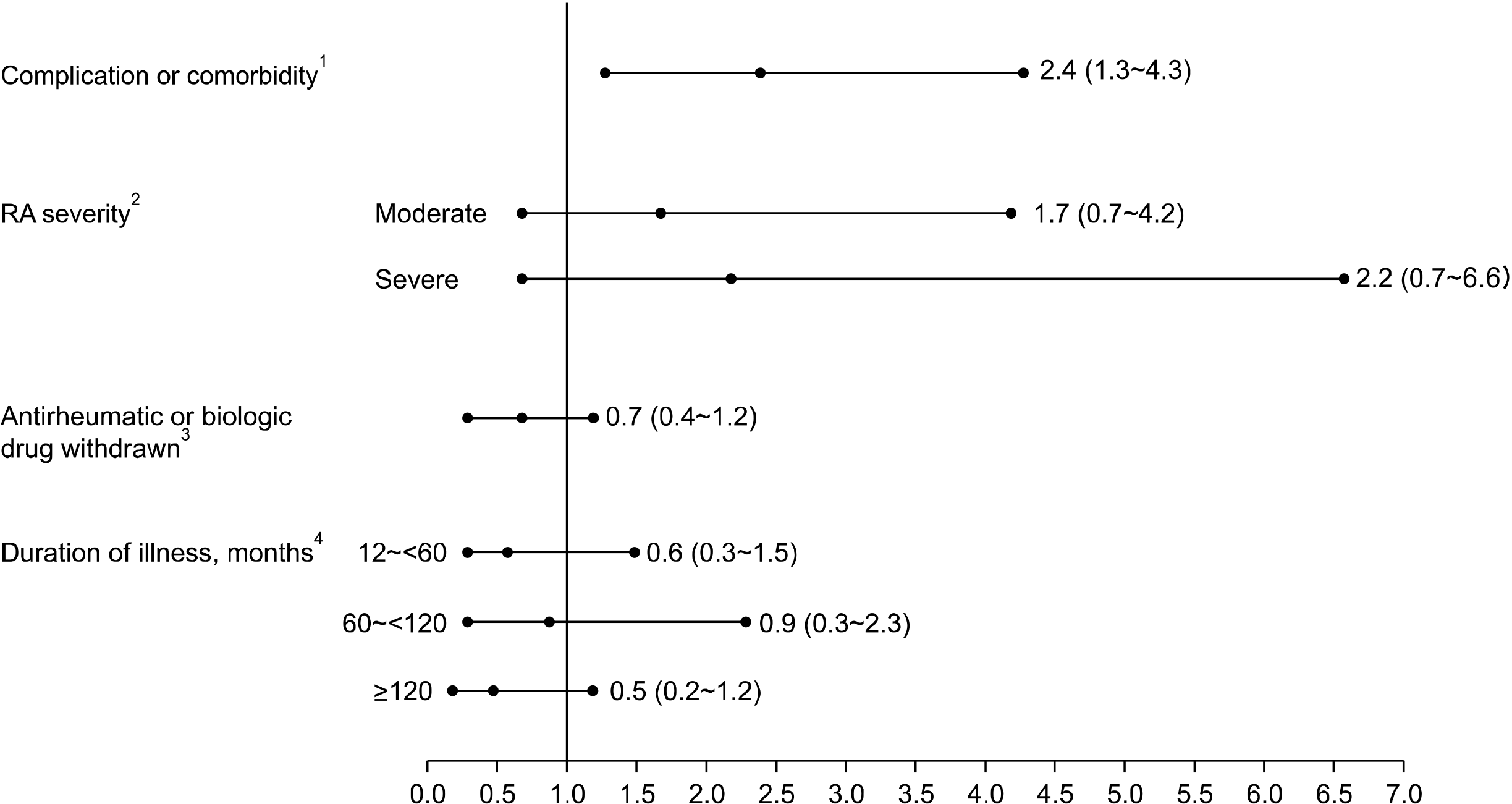
1) Analysis of factors associated with AEs
Figure 1
Surveillance study in LN
Table 3
ADR: adverse drug reaction, AE: adverse event, LN: lupus nephritis, NR: not reported, SAE: serious adverse event, SLE: systemic lupus erythematosus.*Includes AEs classified as certainly, probably/likely, or possibly related to tacrolimus, or thoserecorded as conditional/unclassified or not assessable/unclassifiable. †AEs leading to discontinuation were: nausea, diarrhea, abdominal pain, vomiting, albuminuria, azotemia, decreased creatinine clearance, hematuria, pneumonia, fever, pain, hyperglycemia, headache, dizziness, eczema, alopecia, unspecified infection, auto-antibody response, cellulitis, hypertension, and thrombocytopenia. 98 AEs were reported in 64 patients.
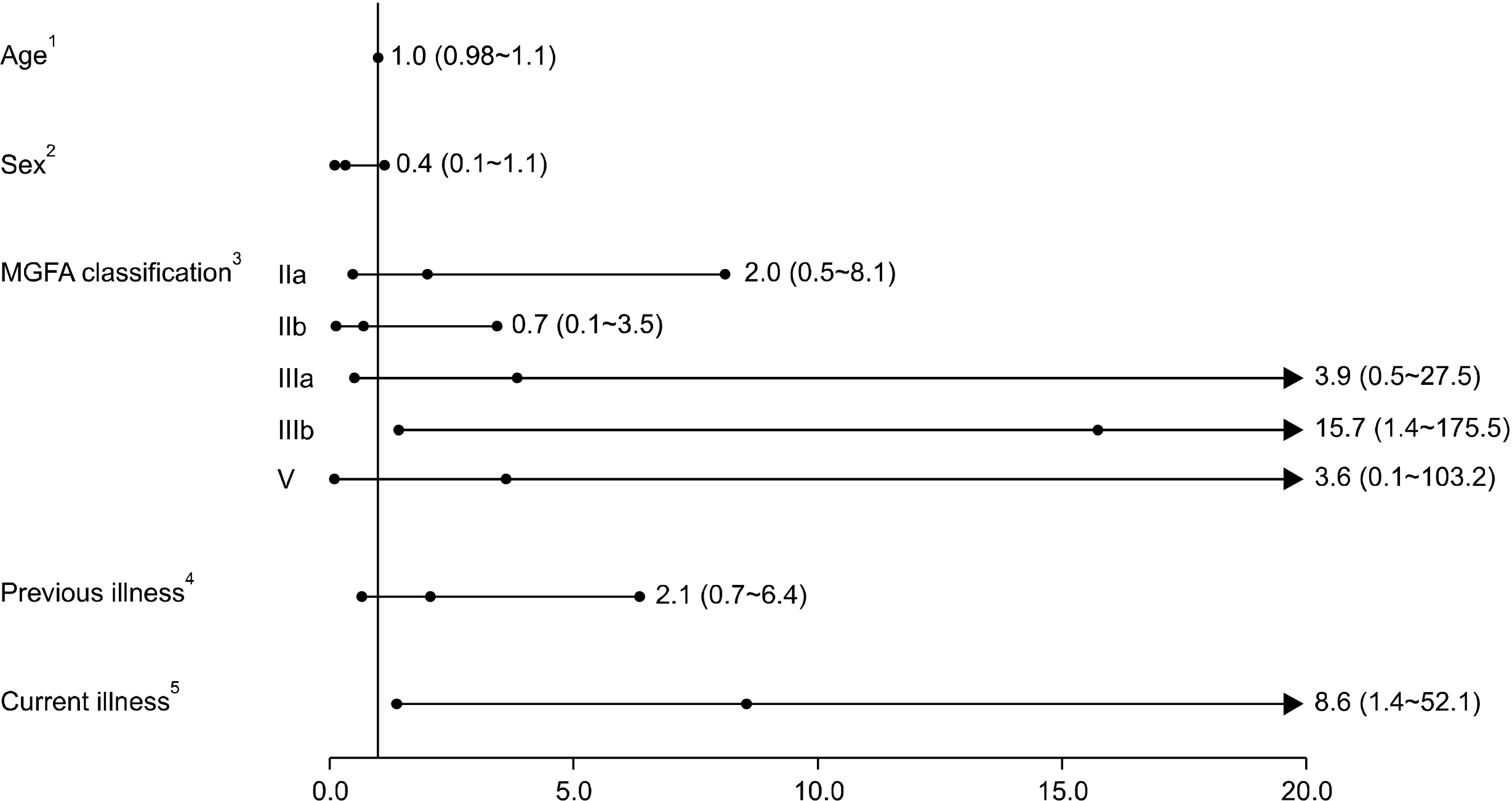
1) Analysis of factors associated with AEs
Figure 2
Surveillance study in MG
Table 4
ADR: adverse drug reaction, AE: adverse event, MG: myasthenia gravis, NR: not reported, SAE: serious adverse event. *Includes AEs classified as certainly, probably/likely, or possibly related to tacrolimus, or those recorded as conditional/unclassified or not assessable/unclassifiable. †AEs leading to discontinuation were: diarrhea, heartburn, dyspepsia, nausea, alopecia, hyperglycemia, herpes zoster, headache, and cardiac arrest. ‡All AEs reported are shown. 44 AEs were reported in 31 patients.
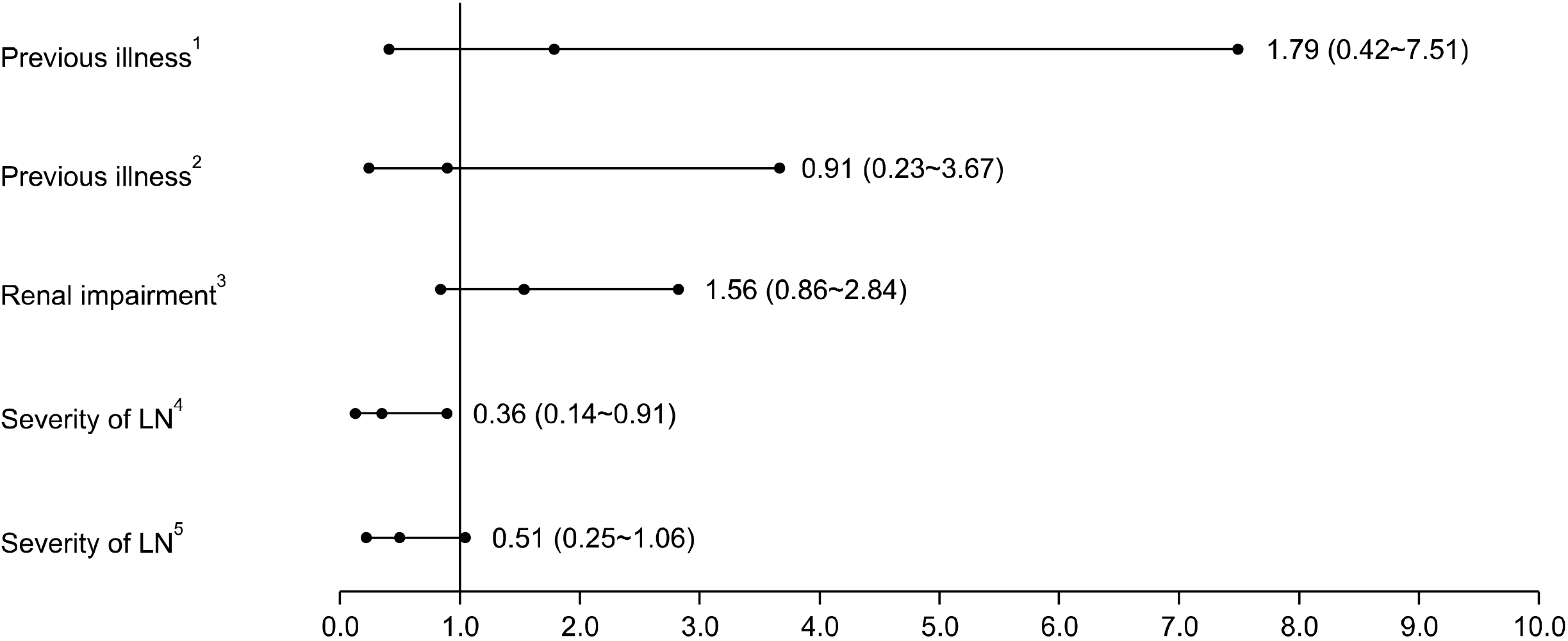
1) Analysis of factors associated with AEs
Figure 3




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download