Abstract
Hemangioma is a benign tumor characterized by the proliferation of blood vessels. Although it often appears in soft tissues, its occurrence in bone tissue, particularly the mandible, is extremely rare. A 32-year-old female sought attention at the dental clinic complaining of a painless swelling in the posterior region of the left side of the mandible. A panoramic radiograph and computed axial tomography scan were taken, showing honeycomb and sunburst images, respectively, in the affected area. The patient underwent a biopsy, which led to the diagnosis of intraosseous hemangioma. Having assessed the characteristics of the lesion, it was decided to perform complete excision including safety margins, followed by an iliac crest bone graft to reconstruct the mandible. Awareness of the possible clinical and radiographic presentations of intraosseous hemangioma is considered important, as non-diagnosis could have severe consequences given its possible relation to dental structures.
Go to : 
Intraosseous hemangioma is a benign tumor of endothelial origin that represents only 0.5%-1% of all benign bone tumors and is characterized by the proliferation of blood vessels1.
Although hemangiomas are relatively frequent in soft tissues, their occurrence in bone tissue is quite rare and usually located in the spinal column or skull1,2.
Hemangioma may present at any age but is more frequent in adults between the second and fifth decades of life and, according to most research, has a clear predilection for females1-6.
Diagnosis of this tumor is very complicated, as it is generally asymptomatic until it reaches a certain size and the patient starts to notice a slow-growing swelling, which may be accompanied by occasional pain, speech difficulties, or esthetic deformity2.
Its radiographic appearance is non-specific, making diagnosis even more difficult. It can appear as a unilocular or multilocular radiolucency, reticular pattern, honeycomb-like pattern, sunburst-like image, or as a set of vertical striations similar to the bars of a prison cell; it may even appear as a radiopaque area1-3,7,8.
Given its low incidence and non-specific clinical and radiographic forms of presentation, intraosseous hemangioma constitutes a real diagnostic challenge.
Go to : 
A female patient aged 32 years came to the clinic because of a swelling of hard consistency in the left hemimandible. She reported first noticing the lump approximately a year previously.
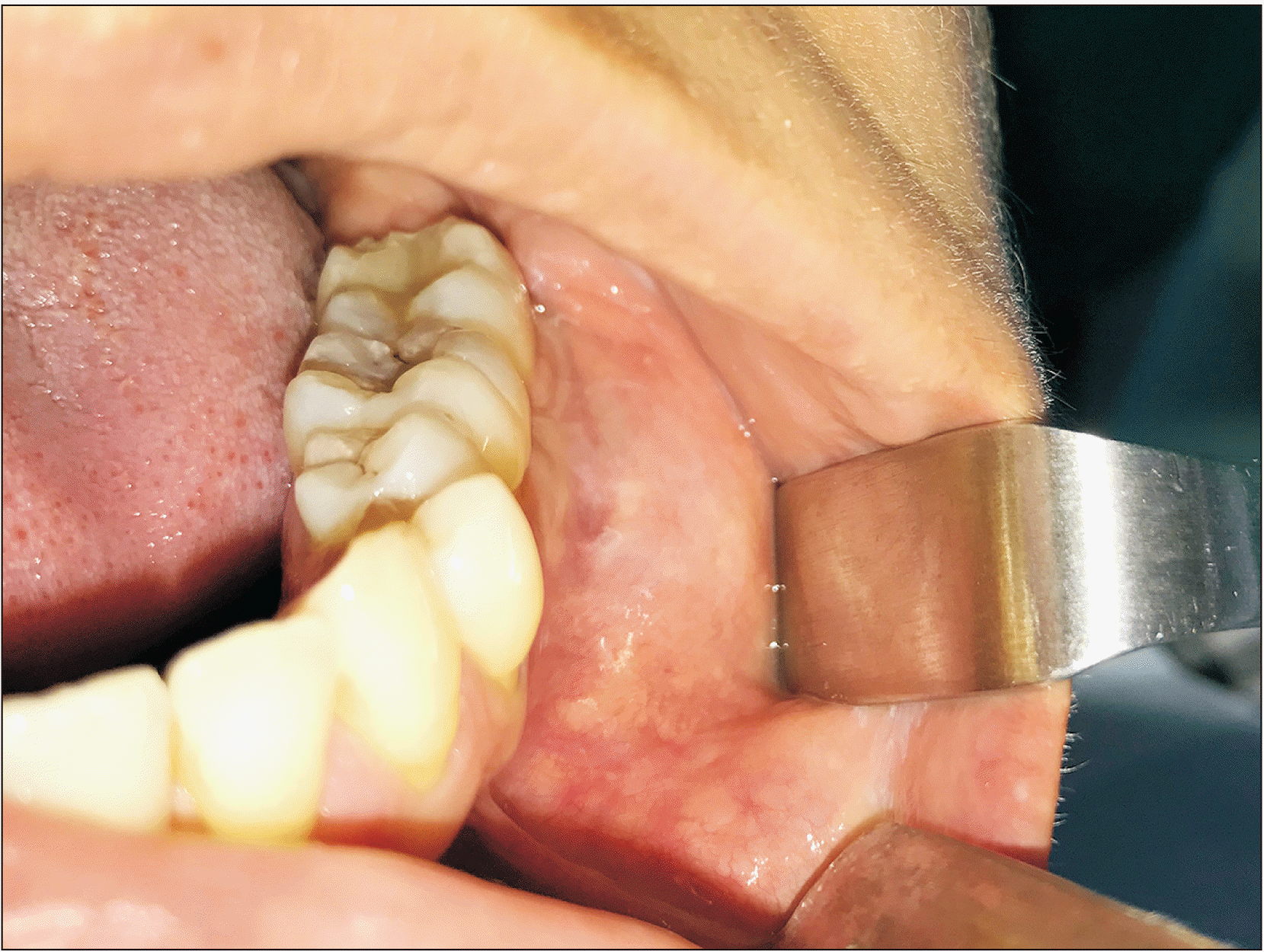
Upon exploration, an increased volume in the vestibular area adjacent to teeth #35, #36, and #37 was observed, which was hard but painless on palpation, without mucosal alteration, and practically indiscernible in extraoral examination.(Fig. 1) No dental mobility or pain on percussion were observed.
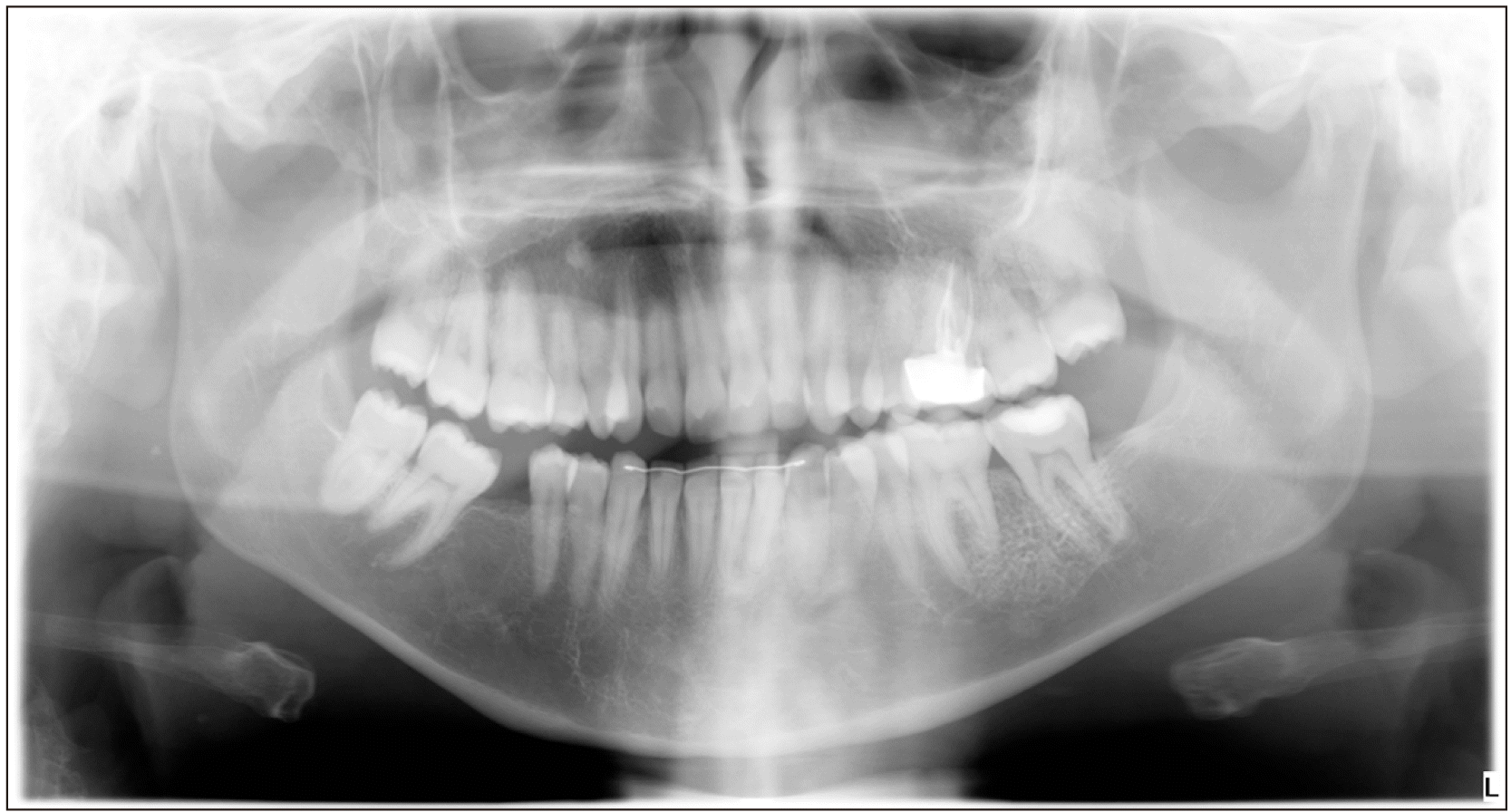
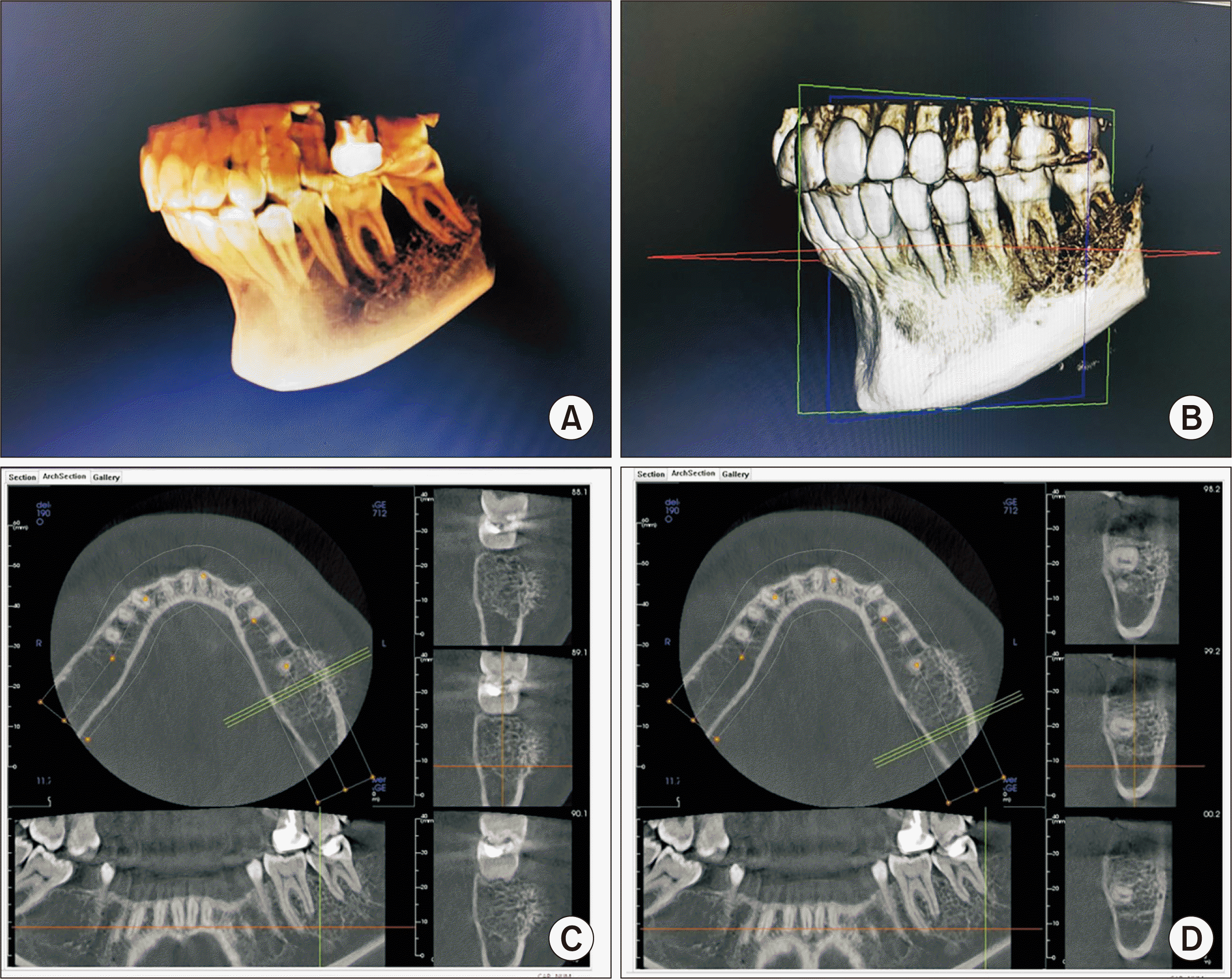
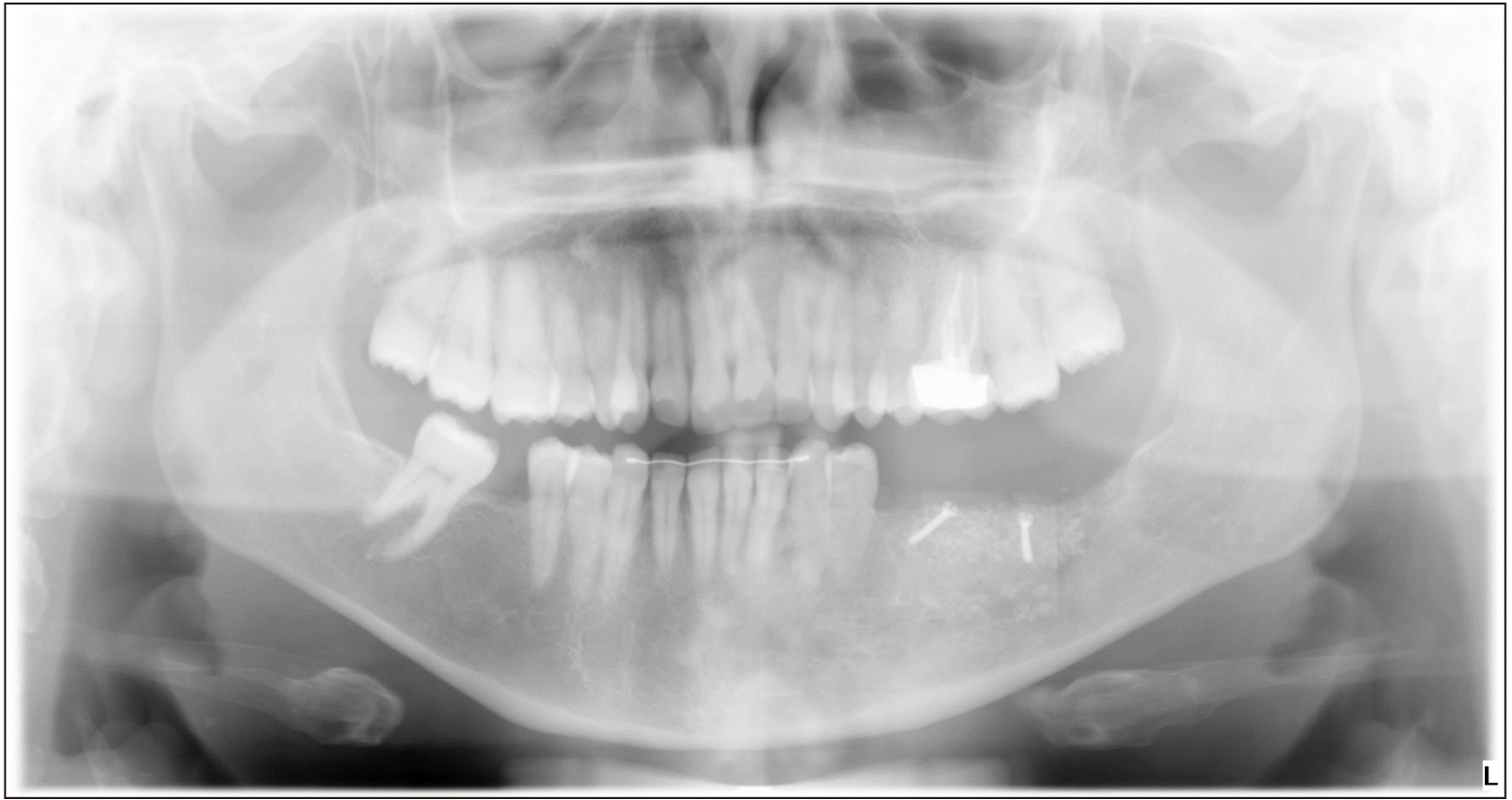
A panoramic radiograph was taken, which showed a reticular pattern with a honeycomb appearance in the region of the swelling.(Fig. 2) This was compared with an earlier radiograph taken two years earlier, in which the lesion went unnoticed, as it was not yet clinically present. Then, a computed tomography (CT) scan was captured, which provided a sunburst-like image. The lesion measured approximately 30 mm×25 mm, which did not affect the lingual cortical bone plate but did affect vestibular cortical bone, presenting reactive bone spicules.(Fig. 3)
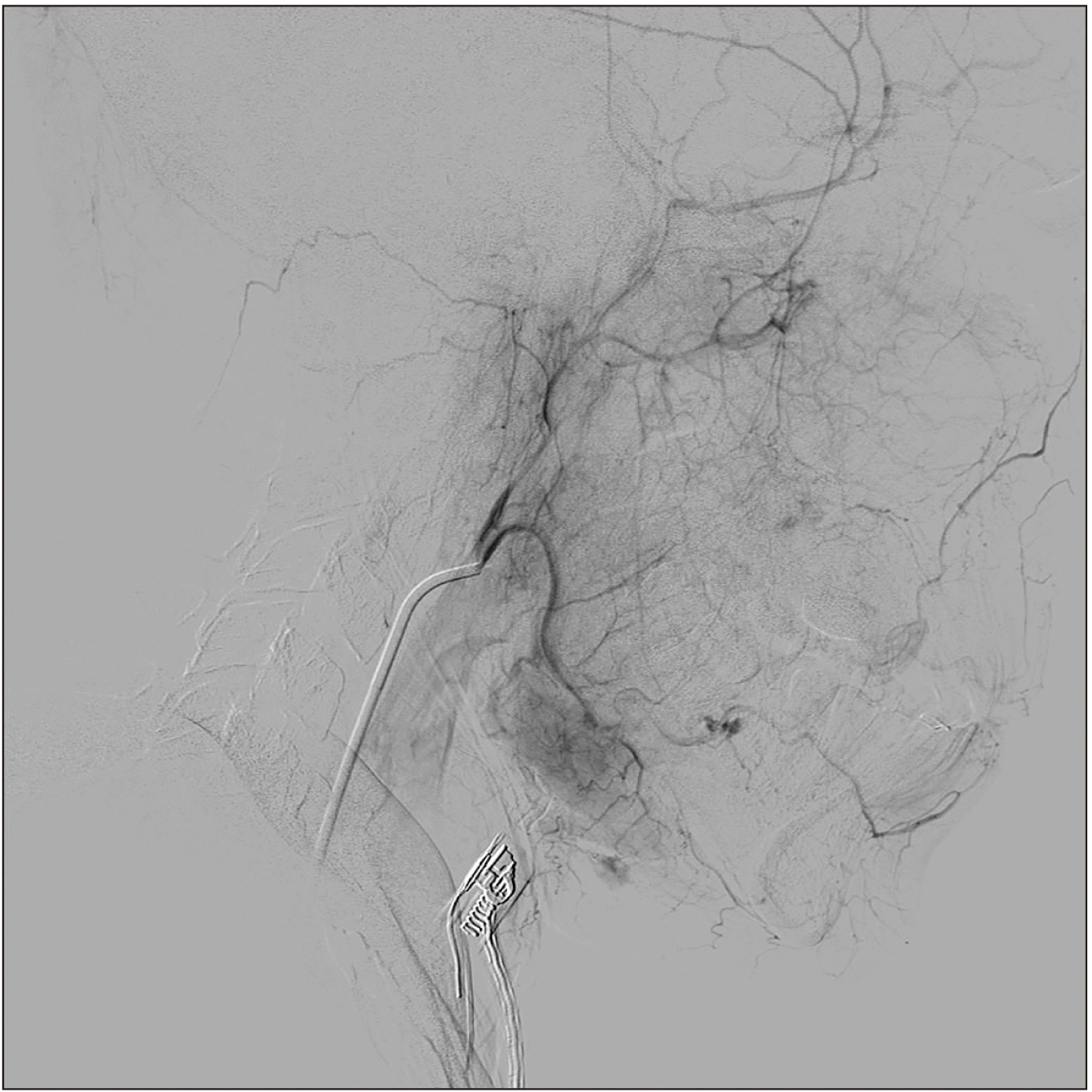
It was decided to perform an intrabony incisional biopsy to obtain a definitive diagnosis, applying appropriate safety measures to avoid hemorrhage. Before this biopsy surgery, magnetic resonance imaging (MRI) and angiography were performed to determine the extent of tumor growth into the soft tissue, assess the blood flow, and identify nutrient vessels.(Fig. 4)
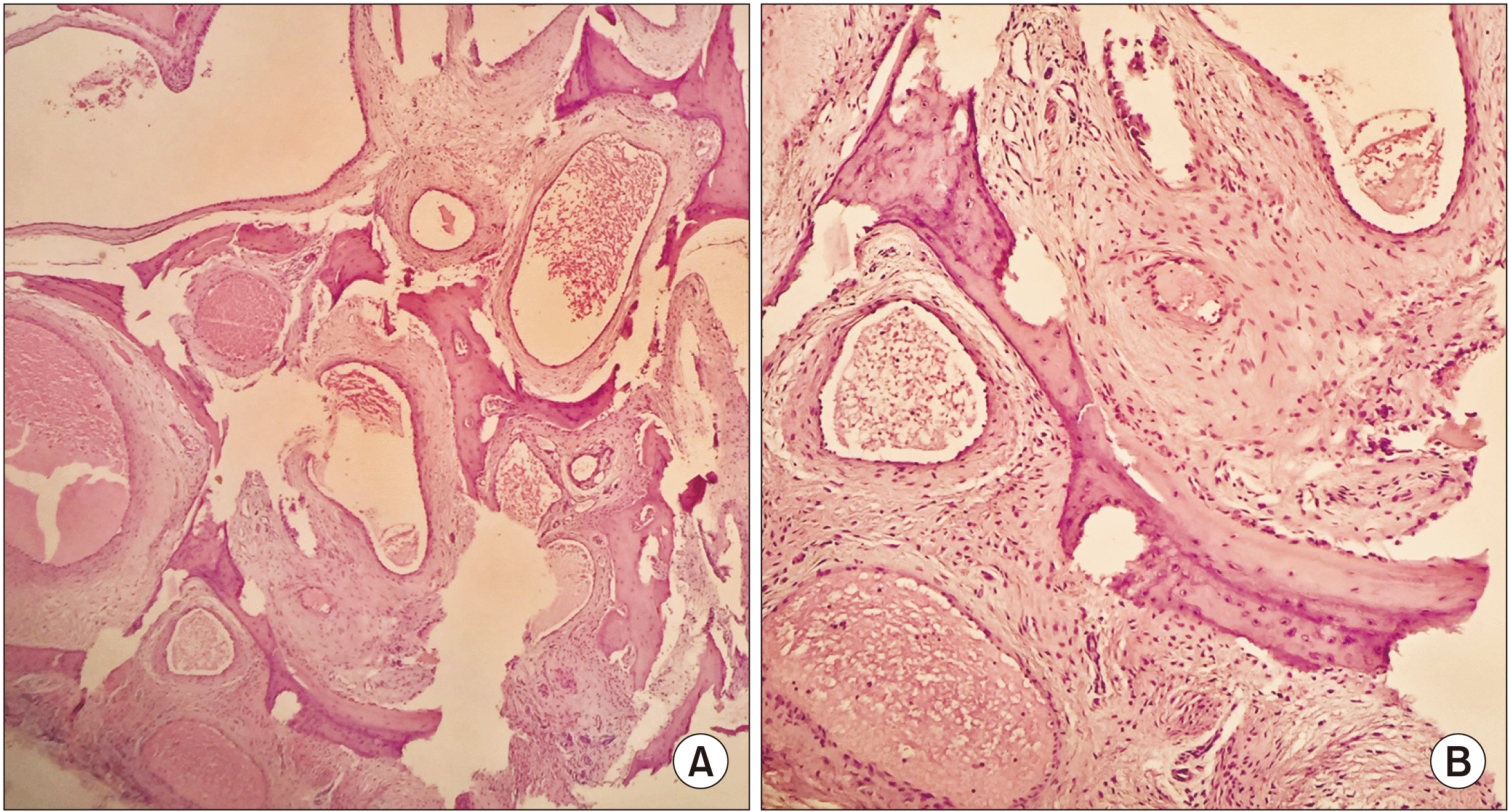
Anatomopathological analysis revealed the presence of bone trabeculae and spicules, and between them, numerous vascular structures were identified with muscular walls and dilated spaces separated from one another by fibrous tissue. No signs of malignancy were observed. The diagnosis was an intraosseous cavernous hemangioma of the mandible.(Fig. 5)
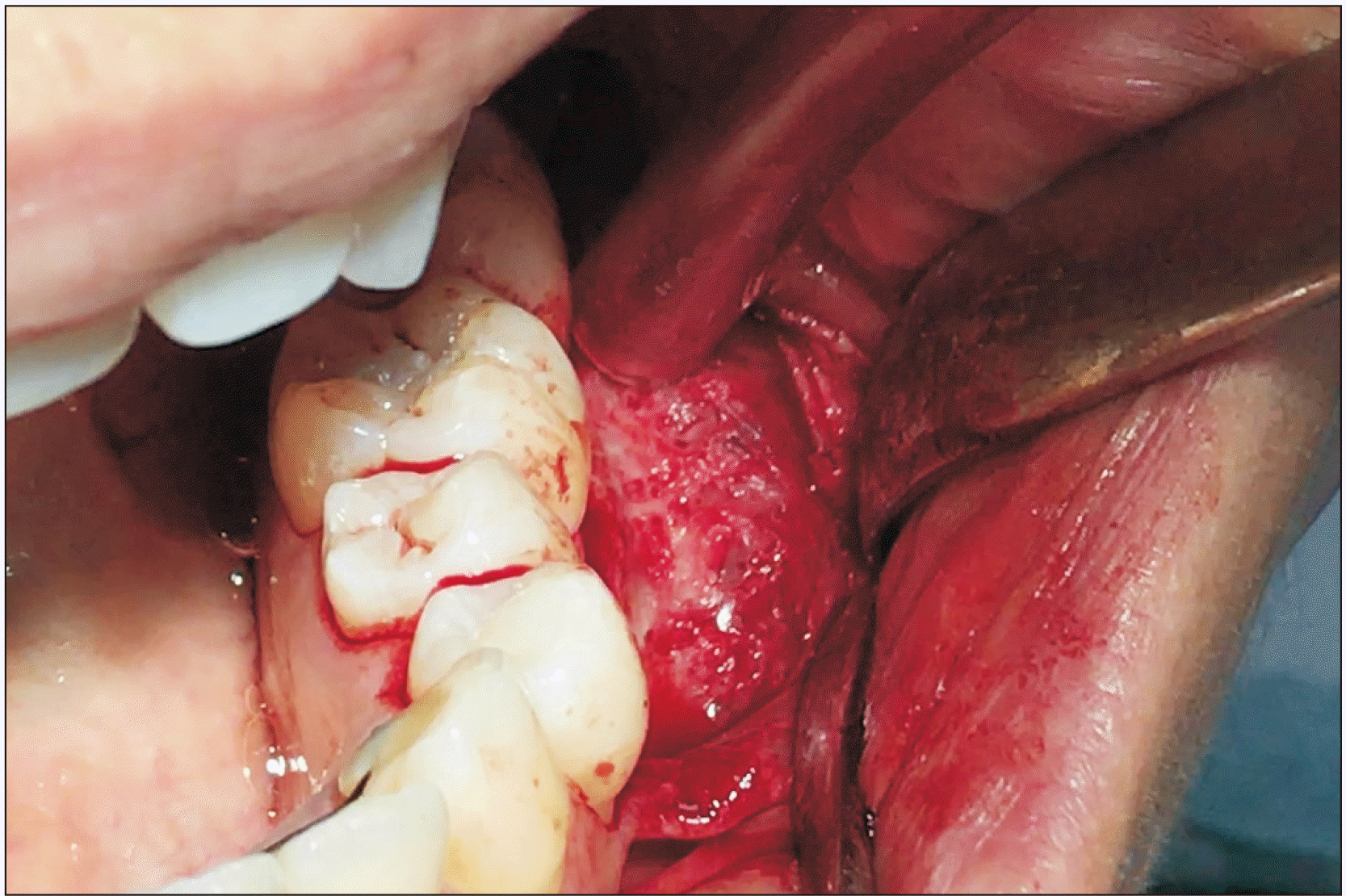
Intraoperatively, the lesion presented a moth-eaten appearance and was filled with vascular soft tissue.(Fig. 6) Given the patient’s age, the treatment of choice was to remove the hemangioma with safety margins, followed by an iliac bone graft to reconstruct the mandible. After surgery, the patient reported anesthesia of the lower-left dental nerve, of which she had been warned previously.
A radiograph taken one year after surgery showed no signs of relapse.(Fig. 7) The anesthesia of the lower-left dental nerve persists, and the patient is currently scheduled to receive dental implants.
Go to : 
Hemangiomas are benign vascular neoplasias characterized by an abnormal proliferation of blood vessels. They can occur in any vascularized tissue, including skin, subcutaneous tissue, muscle, and bone; intraosseous hemangiomas represent only 1% of all hemangiomas2,9. When they are located in bone tissue, they generally appear in the spinal column or skull; hemangioma is extremely rare in the jaw. Regarding jawbone hemangioma, most researchers agree that there is a higher incidence in the mandible than in the maxilla6-9. In an investigation by Donohue et al.1, two-thirds of jaw hemangioma presented in the mandible and one-third in the maxilla, and they tended to show a special predilection for the mandibular premolar and molar regions (as the present case substantiates), followed by the ascending ramus, condyle, and palate1,8,10.
Based on their appearance under the microscope, hemangioma can be classified into three types: cavernous, capillary, and mixed. Cavernous hemangiomas show large vascular spaces with little fibrous tissue stroma, while the vascular spaces are small, and stroma is abundant in the capillary type. The mixed type shows features of both the other types3,4. Capillary hemangioma, characterized by the presence of tiny, fine blood vessels, is usually present at birth, affecting soft tissue, and usually decreases in size during the post-natal phase. Most cavernous hemangioma appears in adulthood and represents the majority of intraosseous hemangiomas in the maxillofacial massif2, as in the present case. Nevertheless, although rare, a few cases of capillary intraosseous hemangioma have been reported, for example, by Dereci et al.11.
Intraosseous hemangiomas are also classified in terms of their site of origin as peripheral or central hemangiomas. The peripheral type originates in periosteum blood vessels with a growth pattern towards medullar bone, while the central type originates in medullar bone and extends towards cortical bone4,5.
The origin of intraosseous hemangiomas is a subject of debate. They have been regarded as real neoplasias, in which the dental artery of the lower dental canal is the origin of the lesion, which would explain its greater predilection for mandibular premolar and molar regions1 in addition to why the canal is widened in many of these cases5.
Other authors consider them to be hamartomas, the result of intraosseous mesoderm cell proliferation that undergoes endothelial differentiation1,4,5,10.
Intraosseous hemangiomas have also been considered congenital lesions originating in the bone medulla, as remains of active hematopoietic medulla have been detected in posterior areas of the mandible, the areas where hemangiomas most often occur1.
Their pathogenesis remains unknown. Some authors note localized trauma as a possible cause as it is not uncommon for hemangioma patients to present antecedents of trauma2,3.
Intraosseous hemangiomas grow very slowly and remain clinically indiscernible until they reach a size noticeable by the patient, usually as a swelling of generally hard consistency, as in the present case. Nevertheless, cases of hemangioma of soft consistency have been described12. At times they may be accompanied by pain, as in the case reported by Dhiman et al.10; speech difficulties, as reported by Elif et al.2; reduced mouth opening, as mentioned by Donohue et al.1; a throbbing sensation, bleeding in the gingival sulcus, and a reddish coloration, as reported by Fernández et al.8; paresthesia, as in the case described by Eliot and Castle13; root resorption4,8-10; dental mobility; and dental exfoliation1,3,4. Reports even include a case of retention of an upper canine and its displacement towards the zygomatic bone, probably provoked by the presence of an intraosseous hemangioma14. The relevance of hemangioma lies in its proximity to dental structures, involving a high risk of hemorrhage when a dental extraction is performed.
In addition to the enduring absence of clinical manifestations, their radiographic appearance is not pathognomonic and may be very similar to a range of other bone lesions, making diagnosis complicated. Vascular anomalies are known to be of very similar appearance radiographically1. The absence of clinical signs in the present case meant that the lesion went unnoticed for several years, although an earlier panoramic radiograph showed an altered bone pattern.
Among the possible radiographic manifestations of intraosseous hemangioma, the most characteristic is a honeycomb pattern in panoramic radiographs, an image that, according to Sepulveda et al.4, appears in 50% of cases. A sunburst image characteristically appears in CT scans due to the presence of reactive bone spicules1,2. Both types of images appeared in the panoramic radiograph and CT captured in the present case. However, these images do not always appear, and hemangioma often appears as just a unilocular1,9 or multilocular12 radiolucent image similar to cystic lesions. Cases of radiopaque appearance have also been described2,9.
Sometimes, the lower dental canal appears wider than usual, suggesting the vascular origin of the lesion5,6,12.
When making a diagnosis, osteomas, giant cell tumor, fibrous dysplasia, dermoid tumor, chondrosarcoma, osteogenic sarcoma, dental cyst, aneurysmal or solitary bone cyst, ameloblastoma, and myxoma are among the entities that must be included in differential diagnosis1,2,5,13,15,16. Certain diagnoses can only be obtained by biopsy, which should be performed with caution due to the high risk of hemorrhage; fine-needle aspiration biopsy reduces this risk1.
Deciding on the most effective treatment should be determined by the tumor’s location, size, and blood supply3,6. Among the recommended radiographic tests are panoramic radiographs, computed axial tomography (CAT) scans, nuclear magnetic resonance (NMR), and conventional angiography2,3. The CAT scan is considered the most useful imaging technique for assessing details relating to trabecular and cortical bone2. NMR is the most effective method for identifying the depth and extent of the lesion in soft tissues1,2, as well as its blood flow5. The injection of a contrast medium in both CAT and NMR makes it possible to assess cortical involvement and the extent of the lesion4. Angiography imparts visualization of the main vessels supplying the lesion3.
The ideal choice of treatment must consider the patient’s age; medical state; and the tumor’s clinical manifestations, size, extent, and blood supply into account.
Most authors agree that the most effective treatment for preventing recurrence is complete resection of the tumor2,10, recommending block excision of the lesion including a safety margin of surrounding healthy bone and nutrient vessel ligation if present. In most cases, surgical excision of the lesion will require reconstruction of the eliminated area. To do this, the use of titanium plates is proposed, which may be combined with grafts harvested from the ribs, iliac crest, or other bone sites10,12,17.
As alternatives to complete tumor resection, other treatments have been proposed, such as radiotherapy, sclerotherapy, embolization, and ligation of the external carotid artery2-4.
Today, radiotherapy is reserved for cases in which surgery is not a viable option because of potential adverse effects, such as telangiectasia, delayed bone and dental growth, tissue necrosis, or malignant degeneration12.
Sclerotherapy is not considered effective because of the lesion’s bone affectation12 and could be dangerous in any case due to the proximity to intracranial circulation3.
The use of isolated embolization has been described as successful in the treatment of intraosseous hemangioma in a case report by Sepulveda et al.4, but its efficacy is only palliative in many cases. Embolization is more commonly used preoperatively to reduce the risk of hemorrhage during surgery1. Although many authors consider this unnecessary, providing excision should include adequate safety margins2, as in the case described here. It is also important to take into account that embolization may be subject to complications, including atheroma release, catheter breakage, vessel breakage, hemiplegia, blindness, facial paralysis, allergic reactions, and avascular necrosis of the mandible6.
External carotid artery ligation is not an effective treatment either, as the formation of collateral circulation derived from the collateral external carotid artery or the ipsilateral internal carotid artery does not result in the loss of tumor vascularization3,6.
Clinical observation of intraosseous hemangioma is only indicated for asymptomatic patients with minimal or no facial deformity1,4,5.
Malignant transformation of intraosseous hemangioma is extremely rare and has only been reported following radiotherapy1.
Generally, the prognosis of intraosseous hemangioma is good once the lesion has been removed and bone regeneration is underway3. However, although uncommon, relapses have been reported2,9.
Intraosseous hemangioma of the mandible is a rare benign bone tumor. The difficulty of diagnosing this lesion is due to not only its low incidence but also to its multiple and non-specific forms of clinical and radiographic presentation. Early diagnosis is important because even though benign, early treatment is advised to avoid major complications derived from local growth, which can worsen prognosis.
Go to : 
Notes
Authors’ Contributions
A.G.C. operated the case and helped in crafting the study design. M.I.S.J. and J.C.B.B. participated in study design and coordination and helped to draft the manuscript. R.A.O. contributed to the study design and wrote the manuscript. All authors contributed to data acquisition and analysis, and they also read and approved the final manuscript.
Go to : 
References
1. Donohue CA, de la Torre MA, de la Torre MG, Sánchez AJG, López MJA, Guzmán GDA, et al. 2016; Hemangioma intraóseo: reto diagnóstico. Presentación de un caso y revisión de la literatura. Rev ADM. 73:39–43. Spanish.
2. Elif B, Derya Y, Gulperi K, Sevgi B. 2017; Intraosseous cavernous hemangioma in the mandible: a case report. J Clin Exp Dent. 9:e153–6.
https://doi.org/10.4317/jced.52864
. DOI: 10.4317/jced.52864. PMID: 28149481. PMCID: PMC5268109.

3. Treviño AMG, Valdés MJ, Martínez MHR, Moreno TMG, Rivera SG. 2016; Hemangioma intraóseo de la mandíbula. Reporte de un caso clínico. Rev ADM. 73:96–8. Spanish.
4. Sepulveda I, Spencer ML, Platin E, Trujillo I, Novoa S, Ulloa D. 2013; Intraosseous hemangioma of the mandible: case report and review of the literature. Int J Odontostomat. 7:395–400.
https://doi.org/10.4067/S0718-381X2013000300010
.

5. Gómez Oliveira G, García-Rozado A, Luaces Rey R. 2008; Intraosseous mandibular hemangioma. A case report and review of the literature. Med Oral Patol Oral Cir Bucal. 13:E496–8. PMID: 18667983.
6. Luaces Rey R, García-Rozado González A, López-Cedrún Cembranos L, Ferreras Granado J, Charro Huerga E. 2006; Intraosseous hemangioma of the mandible. An intraoral approach. Rev Esp Cirug Oral y Maxilofa. 28:195–9.
7. Zlotogorski A, Buchner A, Kaffe I, Schwartz-Arad D. 2005; Radiological features of central haemangioma of the jaws. Dentomaxillofac Radiol. 34:292–6.
https://doi.org/10.1259/dmfr/37705042
. DOI: 10.1259/dmfr/37705042. PMID: 16120879.

8. Fernández LR, Luberti RF, Domínguez FV. 2003; Radiographic features of osseous hemangioma in the maxillo-facial region. Bibliographic review and case report. Med Oral. 8:166–77. PMID: 12730651.
9. Chandra SR, Chen E, Cousin T, Oda D. 2017; A case series of intraosseous hemangioma of the jaws: various presentations of a rare entity. J Clin Exp Dent. 9:e1366–70.
https://doi.org/10.4317/jced.54285
. DOI: 10.4317/jced.54285. PMID: 29302291. PMCID: PMC5741852.

10. Dhiman NK, Jaiswara C, Kumar N, Patne SC, Pandey A, Verma V. 2015; Central cavernous hemangioma of mandible: case report and review of literature. Natl J Maxillofac Surg. 6:209–13.
https://doi.org/10.4103/0975-5950.183866
. DOI: 10.4103/0975-5950.183866. PMID: 27390499. PMCID: PMC4922235.

11. Dereci O, Acikalin MF, Ay S. 2015; Unusual intraosseous capillary hemangioma of the mandible. Eur J Dent. 9:438–41.
https://doi.org/10.4103/1305-7456.163236
. DOI: 10.4103/1305-7456.163236. PMID: 26430377. PMCID: PMC4570000.

12. Chetan BI, Shruthi DK, Karthik B. Sharmila. 2015; Diagnostic and surgical aspects of central hemangioma of mandible: a surgical approach for the reconstruction of mandible. J Int Oral Health. 7:56–8. PMID: 25709370. PMCID: PMC4336663.
13. Eliot CA, Castle JT. 2010; Intraosseous hemangioma of the anterior mandible. Head Neck Pathol. 4:123–5.
https://doi.org/10.1007/s12105-010-0170-x
. DOI: 10.1007/s12105-010-0170-x. PMID: 20512636. PMCID: PMC2878621.

14. Kalsi H, Scannell J. 2013; Unusual presentation of an intraosseous hemangioma of the maxilla and displaced canine. Int J Clin Pediatr Dent. 6:124–6.
https://doi.org/10.5005/jp-journals-10005-1203
. DOI: 10.5005/jp-journals-10005-1203. PMID: 25206206. PMCID: PMC4086593.

15. Kaya B, Işılgan SE, Cerkez C, Otrakçı V, Serel S. 2014; Intraosseous cavernous hemangioma: a rare presentation in maxilla. Eplasty. 14:e35. PMID: 25328568. PMCID: PMC4194598.
16. Handa H, Naidu GS, Dara BG, Deshpande A, Raghavendra R. 2014; Diverse imaging characteristics of a mandibular intraosseous vascular lesion. Imaging Sci Dent. 44:67–73.
https://doi.org/10.5624/isd.2014.44.1.67
. DOI: 10.5624/isd.2014.44.1.67. PMID: 24701461. PMCID: PMC3972408.

17. Omeje K, Efunkoya A, Amole I, Akhiwu B, Osunde D. 2014; A two-year audit of non-vascularized iliac crest bone graft for mandibular reconstruction: technique, experience and challenges. J Korean Assoc Oral Maxillofac Surg. 40:272–7.
https://doi.org/10.5125/jkaoms.2014.40.6.272
. DOI: 10.5125/jkaoms.2014.40.6.272. PMID: 25551091. PMCID: PMC4279977.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download