I. Introduction
II. Materials and Methods
1. Literature search
2. Study inclusion and exclusion criteria
3. Search strategy
4. Study selection
5. Risk of bias assessment
Table 1
6. Data management and extraction
III. Results
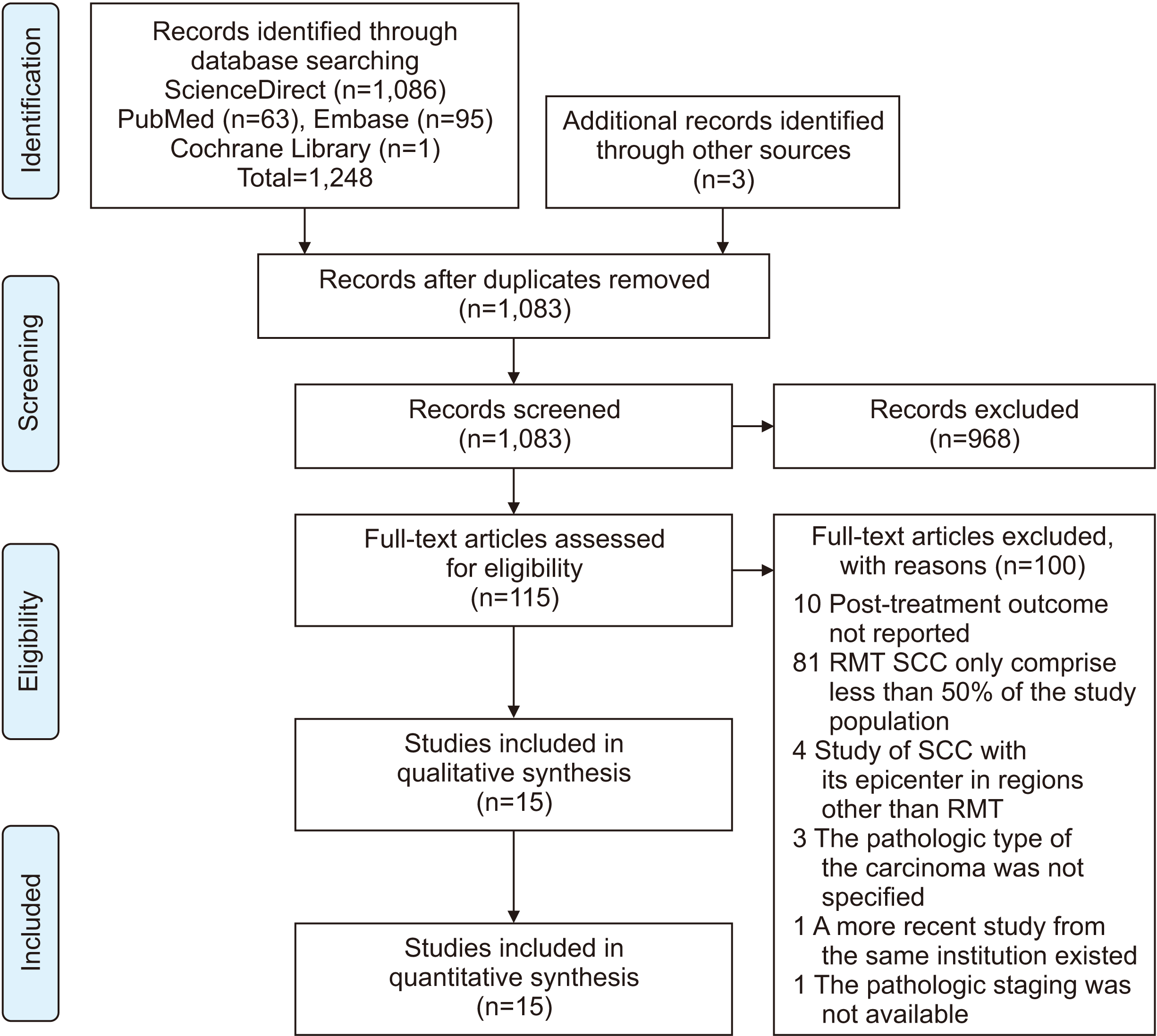 | Fig. 1Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart. (RMT: retromolar trigone, SCC: squamous cell carcinoma) |
Table 2
(RT: radiotherapy, CCRT: concurrent chemoradiotherapy, PMMC: pectoralis major myocutaneous flap, ALT: anterolateral thigh flap, STSG: split thickness skin graft, FFF: fibula free flap, RFFF: radial forearm free flap, mRND: modified radical neck dissection, RND: radical neck dissection, PEG: percutaneous endoscopic gastrotomy)
1. Eligibility and quality assessment
Table 3
| Study | Study design (8✓) |
Study measurements (4✓) |
Statistical analysis (4✓) |
Total | Risk of bias | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||||
| A | B | C | D | E | F | G | H | I | J | K | |||||
| Deo et al.9 (2013) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓×× | ✓✓ | ✓× | ✓ | ✓ | ✓× | 12 | Moderate | ||
| Huang et al.10 (2001) | ✓ | ✓ | ✓ | ✓ | × | ✓×× | ✓✓ | ×× | ✓ | ✓ | ✓× | 10 | Moderate | ||
| Kowalski et al.11 (1993) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓×× | ✓✓ | ✓✓ | × | ✓ | ✓✓ | 13 | Moderate | ||
| Petruzzelli et al.13 (2003) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓×× | ✓✓ | ×× | ✓ | ✓ | ✓× | 11 | Moderate | ||
| Binahmed et al.12 (2007) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓×× | ✓✓ | ✓✓ | ✓ | ✓ | ✓× | 13 | Moderate | ||
| Bayman et al.14 (2010) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓×× | ✓✓ | ✓✓ | × | ✓ | ✓✓ | 13 | Moderate | ||
| Factor et al.15 (2020) | ✓ | ✓ | × | ✓ | × | ✓×× | ✓✓ | ×× | × | ✓ | ✓✓ | 9 | Low | ||
| Ayad et al.16 (2005) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓×× | ✓✓ | ✓✓ | ✓ | ✓ | ✓× | 13 | Moderate | ||
| Hitchcock et al.17 (2015) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓×× | ✓✓ | ✓✓ | × | ✓ | ✓× | 12 | Moderate | ||
| Nishi et al.18 (2018) | ✓ | ✓ | ✓ | × | ✓ | ✓×× | ✓✓ | ✓× | × | ✓ | ✓× | 10 | Moderate | ||
| Byers et al.19 (1984) | ✓ | ✓ | ✓ | ✓ | × | ✓×× | ✓✓ | ✓✓ | × | × | ×× | 9 | Low | ||
| Hao et al.20 (2006) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓×× | ✓✓ | ✓✓ | × | ✓ | ✓✓ | 13 | Moderate | ||
| Demir and Öztürk Yanaşma21 (2020) | ✓ | ✓ | × | ✓ | ✓ | ✓×× | ✓✓ | ✓× | ✓ | ✓ | ✓× | 11 | Moderate | ||
| Rizvi et al.22 (2018) | ✓ | ✓ | ✓ | ✓ | × | ✓×× | ✓✓ | ✓✓ | × | ✓ | ✓✓ | 12 | Moderate | ||
| Faisal et al.1 (2017) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓×× | ✓✓ | ✓✓ | × | × | ×× | 10 | Moderate | ||
2. Study characteristics
Table 4
| Study | Study characteristic | Patient characteristic | Conclusion of the study | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Sample | Purpose | Control group |
Median F/U (mo) |
Location | Mean age (yr) | Sex, female (%) | Comorbidity | |||
| Deo et al.9 (2013) | 42 | To review locally advanced RMT SCCs treated with radical surgery and postoperative RT. | - | 20 (4-86) | India | 50 | 28.6 (12/42) | Smoking 78% (33/42) | Good outcomes in RMT SCC can be achieved via aggressive surgical approach with postoperative radiation therapy. | |
| Huang et al.10 (2001) | 65 | To identify the prognostic factors and evaluate the therapeutic outcomes of patients treated with preoperative RT, postoperative RT, and RT alone. | - | - (minimum 5 yr) | USA | 61 (20-80) | 43 (28/65) | Smoking 96% (63/65) | Surgery combined with pre- or postoperative RT offers better LRC and DFS than RT alone for RMT SCC. Lymph node status significantly influences the DFS and distant metastasis rate. | |
| Kowalski et al.11 (1993) | 114 | To report the survival results of 114 consecutive patients who underwent an extended “commando” operation (retromolar operation) from 1960-1991. | - | 25 | Brazil | 55 (30-75) | 8.8 (10/114) | - | Retromolar operation can be performed with acceptable morbidity (5-yr OS 55.3%). Adjunctive modality should be indicated in patients in whom recurrences are likely. | |
| Petruzzelli et al.13 (2003) | 16 | To retrospectively review the oncologic outcomes and QoL of patients who underwent posterior marginal mandibulectomy performed in the management of RMT carcinoma or exposure osteotomy to facilitate access to tongue base. | Tongue base (13/29) | 33.73 | USA | 63.07 (41-78) |
31.3 (5/16) 38.5 (5/13) |
- | Posterior osteotomy of the mandibular ramus is a useful adjunct in the surgical treatment of cancer of the RMT or oropharynx. Negative surgical margins can be obtained, and it does not significantly affect appearance. Patients report deterioration in chewing but are able to maintain a diet of solid food. | |
| Binahmed et al.12 (2007) | 76 | To evaluate outcomes in previously untreated patients with SCCs of the RMT and assess the effects of different treatment modalities on recurrence and survival. | - | 60 | Canada | 67.22±10.3 | 26 (20/76) | Tobacco and/or alcohol use 86% (66/76), DM, HTN, ischemic heart disease were most common. | SCCs of the RMT have a poor survival rate for early-stage disease. Adequate surgical margins can improve survival. | |
| Bayman et al.14 (2010) | 43 | To assess the effects of definitive hypofractionated RT as the primary treatment for SCCs of the RMT and to examine the incidence of ORN from this hypofractionated schedule. | - | 59 | UK | 66 (39-84) | 20.9 (9/43) | - | The hypofractionated regimen is convenient for RMT SCCs and produced comparable outcomes to longer fractionation schedules without an increase in late toxicity. | |
| Factor et al.15 (2020) | 14 | Determine whether making anatomical distinction has implications for treatment design and clinical outcomes. | Buccal mucosa (13/27) | - | USA | 58 |
14.3 (2/14) 23.1 (3/13) |
Smoking 42.9% (6/14), 61.5% (8/13) Smokeless tobacco: 0%, 15.4% (2/13) Betel nut: 7.1% (1/14), 15.4% (2/13) Alcohol abuse: 7.1% (1/14) |
SCCs of the buccal mucosa have a poor prognosis with rapid in-field failure. RMT cancers have more favorable outcomes. Differentiating tumor origin is important for prognostication and treatment. | |
| Ayad et al.16 (2005) | 46 | Assess the locoregional control and survival rates of patients with RMT SCCs treated with RT and evaluate different factors affecting LRC and survival. | - | 43 (5-217) | France | 62 (38-87) | 32.6% (15/46) | - | Primary RT can be used for small RMT SCCs (T1-T2). For advanced stages without bone invasion, CCRT can increase locoregional and survival rates. | |
| Hitchcock et al.17 (2015) | 110 | To report and compare survival and clinical outcomes of patients treated with curative intent with definitive RT alone or RT combined with surgery. | - | 54 (1-286) | USA | - | 25.5% (28/110) | - | Rates of recurrence in RMT SCCs are high. Surgery combined with RT produces higher rates of LC, DSS, and OS than definitive RT but also results in higher rates of complications. | |
| Nishi et al.18 (2018) | 45 | To evaluate the outcome of treatment for primary SCC of the RMT. | - | 52.4 (2-197) | Japan | 62.4 (43-89) | 15.6 (7/45) | - | Cervical lymph node metastasis is a negative prognostic factor. Marginal mandibulectomy can be selected for patients without distinct bone-marrow infiltration. | |
| Byers et al.19 (1984) | 110 | To describe criteria for treatment selection, pertinent factors affecting the LC and RC of RMT SCC morbidity and survival. | - | - (minimum 2 yr) | USA | 62 (38-85) | 26.4 (29/110) | Smoking (>1 pack/day) 97%, alcoholism 50% | High incidence of second primary and intercurrent disease accounted for high mortality. Single modality was as effective as combined treatment. The preferred operation emphasizes conservatism in the extent of mandibular bone resection and modified radical neck dissection. The use of electrons and photons maximized cancer control and minimized local complications. | |
| Hao et al.20 (2006) | 50 | To report the survival results of 50 consecutive patients with SCCs of the RMT who underwent curative surgery and to identify some prognostic factors that affect survival. | - | 36 (3-106) | Taiwan | 53 (31-80) | 4 (2/50) | - | The maxilla was more apt to be involved in RMT SCCs than the mandible. Deep infiltration of the masticator space and high incidence of bone invasion worsen the prognosis despite aggressive treatment. | |
| Demir and Öztürk Yanaşma21 (2020) | 20 | To present the treatment outcomes and prognostic factors of patients who underwent initial radical surgical resection and postoperative RT or CCRT for primary SCC of the RMT. | - | 26.3 (1-82) | Turkey | 59.4 (27-76) | 25 (5/20) | - | Surgery with negative surgical margins followed by RT/CCRT showed favorable outcomes at early stages. Advanced tumor stage and lymph node metastasis had negative effects on survival. | |
| Rizvi et al.22 (2018) | 4,022 | To validate survival outcomes based on treatment modality for patients with RMT SCC using population-level data. | - | - | USA | 64.6 (16-85) | 31.5 (1,266/ 4,022) | - | RMT SCC has a poor prognosis, though early-stage tumors (stages I-II) have significantly improved survival. Any surgical intervention independently predicted higher survival outcomes. There may be a role for dual modality approaches, particularly for larger tumors. | |
| Faisal et al.1 (2017) | 62 | To share experiences with RMT SCC as the only tertiary care center in a developing country, with attention to clinicopathological outcomes, patterns of failure, and the effects of different treatment modalities on survival. | - | 15 | Pakistan | 53 (18-79) | 41.9 (26/62) | Smoking 35.5% (22/62), Betel nut 30.6% (19/62), Naswar 16.1% (10/62), alcohol 3.2% (2/62) | RMT SCC is a rare entity with late presentation, and advanced stage results in poor outcomes. These tumors have a high risk of occult metastasis, so neck dissection is recommended. Close and involved surgical margins are related to local failure and adverse survival. Surgery+RT improved overall 3-year survival. | |
(F/U: follow-up, RMT: retromolar trigone, SCC: squamous cell carcinoma, RT: radiotherapy, LRC: locoregional control, DFS: disease-free survival, OS: overall survival, QoL: quality of life, DM: diabetes mellitus, HTN: hypertension, ORN: osteoradionecrosis, CCRT: concurrent chemoradiotherapy, LC: local control, DSS: disease-specific survival, RC: regional control)
3. Tumor characteristics
Table 5
| Study | Tumor characteristics | Treatment-related characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Sublocation other than RMT | Stage |
Nodal metastasis (%) |
ENE (+) (%) | Mandibular bone invasion | Intervention | Characteristic of surgery | Positive margin | ||
| Deo et al.9 (2013) | 64.2%, predominantly to buccal & alveolobuccal areas | Only T3-T4 |
50% Level I (31%), Level I+II (19%), skip metastasis (7.1%) |
- | Pathology: 47.6% (7.6% unseen in CT) | Surgery+postoperative RT: 100% (external beam radiation 60-64 Gy/30-32 fractions, 5-6 wk) |
Lip split incision: 100% En bloc hemimandibulectomy: 100% Maxillary alveolectomy: 33.3% Infratemporal fossa clearance: 100% PMMC flap: 95.2% Masseter flap: 4.8% Mandibular reconstruction with titanium plate: 9.5% mRND (spinal accessory nerve): 88.1% RND: 11.9% |
7% | |
| Huang et al.10 (2001) | Anterior tonsillar pillar (34%), soft palate (26%), tongue base (18%) | T1-2: 60%, T3-4: 40% |
33.8% N1 (13.8%), N2A (6.2%), N2B (12.3%), N2C (1.5%) |
18% | 12% (Method not mentioned) |
Surgery+postoperative RT: 60% (46.0-66.6 Gy/1.8-2.9 Gy per daily fraction, 5 day/wk, 60 Co or 4-6 MV) Preoperative RT+surgery: 15% (30-40 Gy, 3-4 wk, 60 Co or 4-6 MV) RT alone: 25% (63-74 Gy) |
- | 33% | |
| Kowalski et al.11 (1993) | 73.7%, oral tongue 14, base of tongue 18, FOM 8, inferior gingiva 61, superior gingiva 18, soft palate 50, hard palate 11 | T1-2: 43%, T3-4: 45.6% Tx: 11.4% |
56.1% N1 (29.8%), N2a (6.1%), N2b (20.2%), N3 (0.9%), Nx (6.1%), Level I (7), Level II (29), Level III (2), Level I+II (20), Level II+IV (2), other (3), not determined (1) |
0.90% | - |
Surgery alone: 40.4% Surgery+postoperative RT: 59.6% (10-70 Gy, median 50 Gy) |
Hemimandibulectomy with midline lip-split: 100% Primary closure: 72.8% PMMC: 8.8% McGregor frontal flap: 5.3% Other: 13.1% Resection, standard: 39.5% Resection, extended: 60.5% RND: 92.1% mRND (spinal accessory nerve): 5.3% SOHND: 2.6% |
3.50% | |
| Petruzzelli et al.13 (2003) | 18.8%, tonsillar pillar, base of tongue | T1-2: 46.2%, T3-4: 53.8% |
44% (38.5%) N1 (4), N2 (1) |
12.50% | Pathology: 15.4% (15.4% unseen in CT) | Surgery+postoperative RT: 100% (total 5,800-6,200 cGy) |
Visor flap or lip-split: 100% Posterior marginal mandibulotomy: 100 % RFFF: 38.5% STSG: 38.5% Platysma flap: 15.4% PMMC: 7.7% mRND: 62.5% SND (I-IV): 37.5% |
- | |
| Binahmed et al.12 (2007) | - | T1-2: 45%, T3-4: 55% |
36.8% cN+: 36% |
- | - |
Surgery alone: 26% Surgery+postoperative RT: 20% Preoperative RT+neck dissection: 3% Palliative Tx: 16% RT alone: 35% |
n=35 Intraoral approach: 14.3% Cheek flap: 85.7% Marginal: 28.6% Segmental: 62.9% None: 8.6% Primary closure: 31.4% |
20% (~2 mm: 22.9%) | |
| Bayman et al.14 (2010) | - | I-II: 51.2%, III-IV: 48.8% | cN+: 30.2% | - | - |
Definitive radical external beam RT: 100% (4 MV/median dose 50 Gy/47.5-55 Gy/16 fractions over 21 days) No chemoradiotherapy |
No surgery | - | |
| Factor et al.15 (2020) | - |
T1-2: 28% (54%), T3-4: 71% (46%) I-II: 7% (46%), III-IV: 92% (54%) |
64% (30%) |
PNI 43% (31%) ECE 36% (8%) |
- |
Surgery+postoperative RT: 100% (66 Gy for positive margin, ENE/60 Gy for PNI or LVSI) CCRT: 50% (46.1%) |
Local resection: 7.1% (15.4%) Composite resection: 92.9% (84.6%) |
57% (31%) | |
| Ayad et al.16 (2005) | 80%, anterior tonsillar pillar (80%), soft palate (59%), lower gum (37%), FOM (30%), buccal mucosa (26%), tongue base (24%), tonsillar fossa (13%), hard palate (11%), mobile tongue (9%), upper gum (2%) | T1-2: 55%, T3-4: 46% |
(Clinical) 46% N1 (33%), N2 (11%), N3 (2%) |
- | 7% (Method not mentioned) |
Preoperative RT+salvage surgery: 26.1% Only RT: 67.4% (external beam RT/ conventional once-daily 2Gy/day, median 66 Gy [60-70]) Neoadjuvant CT: 4.3% Concurrent CT: 2.2% (regimen platinum based) |
- | - | |
| Hitchcock et al.17 (2015) | - | T1-2: 41.8%, T3-4: 58.2% |
20.9% N1 (13), N2a (2), N2b (7), N3 (1) |
- | - |
Surgery+postoperative RT: 55 Surgery+postoperative CCRT: 5 Preoperative RT+surgery: 19 Definitive RT+planned neck dissection: 2 Definitive RT alone: 29 Definitive CCRT: 5 Twice/day: 25, Once/day: 85, definitive RT: median 70 Gy (range, 46-81.6 Gy), pre- and post-operative RT: median 65 Gy (range, 33-75 Gy) |
- | - | |
| Nishi et al.18 (2018) | - | T1-2: 46.7%, T3-4: 53.3% |
42.2% cN+: 48.9% |
- | Pathology: 31.1% (CT not mentioned) |
Surgery alone: 86.6% Surgery+postoperative RT: 6.7% Surgery+CCRT: 6.7% |
Mandibulectomy: 84.4% Marginal: 24.4% Segmental: 60% Primary closure: 15.6% ALT: 28.9% Rectus abdominis: 42.2% FFF: 8.9% Scapula: 2.2% Iliac crest: 2.2% Neck dissection: 86.7% RND: 4.4% SOHND: 60% I-IV: 22.2% |
24.40% | |
| Byers et al.19 (1984) | Soft palate (59.1%), buccal mucosa (42.7%), tonsillar pillar (84.5%), lower gum (60.1%) | T1-2: 63.6%, T3-4: 36.4% |
14.5% cN+: 39% |
13.6% | Pathology: 14% (3.6% unseen in CT) |
Surgery alone: 41.8% Surgery+postoperative RT: 10% (55-60 Gy, 5-6 wk) Preoperative RT+surgery: 2.7% RT alone: 45.5% (17 MeV+18 MV ratio of 1:1-4:1, 17 MeV+60 Co for cN+) |
n=60 Intraoral: 26.7% Lip-split only: 13.3% Marginal: 8.3% Segmental: 51.7% No neck surgery: 6.6% RND: 50% mRND (with SCM, IJV, accessory n. preservation): 16.7% SOHND: 26.7% Primary closure: 25% STSG: 70% Regional flap: 5% |
6.70% | |
| Hao et al.20 (2006) | 84%, buccal membrane (14%), oropharynx (14%) |
T1-2: 48%, T3-4: 52% I: 12%, II: 26%, III: 8%, IV: 54% |
26% | 12% | Pathology: 18% (maxilla: 22%) (CT not mentioned) |
Surgery alone: 42% Surgery+postoperative RT: 46% Surgery+CCRT: 12% Total 6,280 rads (6,120-7,560) - primary site/6,000 rads (4,600-7,400) |
Mandibulotomy: 40% Marginal: 36% Segmental: 24% Inferior maxillectomy: 34% Subtotal maxillectomy: 10% SOHND: 40% mRND: 38% RND: 6% Primary closure: 2% Buccal fat pad flap for <4 cm defects: 12% ALT: 68% FFF: 18% |
2% | |
| Demir and Öztürk Yanaşma21 (2020) | - | T1-2: 35%, T3-4: 65% | 65% | 35% | Pathology: 40% (CT not mentioned) |
Surgery alone: 15% Surgery+RT: 45% Surgery+CCRT: 40% |
Lip-split with composite resection: 70% En-bloc with lip-split: 20% Transoral: 10% Mandibulectomy: 70% Segmental: 40% Marginal: 25% Hemi: 5% Partial maxillectomy: 40% Primary closure: 25% PMMC: 50% ALT: 15% SCM flap: 5% Temporalis m.: 5% Mandibular reconstruction: 10% Neck dissection: 95% SOHND: 10% I-IV: 45% RND: 40% |
0% | |
| Rizvi et al.22 (2018) | - |
T1-2: 65.3%, T3-4: 34.7% I: 24.6%, II: 19.4%, III: 16.9%, IV: 39.0% |
39.50% | - | - |
Surgery alone: 34.2% Surgery+RT: 34.1% Only RT: 23.5% Unknown: 8.2% |
- | - | |
| Faisal et al.1 (2017) | - | T1-2: 5%, T3-4: 8% | 62.5% | 20% LVI: 10% | - |
Surgery alone: 1.8% Surgery+RT: 16.1% Induction CT+surgery+postoperative RT: 4.8% RT alone: 20.9% CCRT: 25.8% Induction CT+CCRT: 30.6% |
n=14 Wide local excision: 42.8% Marginal mandibulectomy: 28.6% Segmental mandibulectomy: 28.6% |
8% (38%) | |
(RMT: retromolar trigone, ENE: extranodal extension, CT: chemotherapy, RT: radiotherapy, PMMC: pectoralis major myocutaneous flap, mRND: modified radical neck dissection, RND: radical neck dissection, FOM: floor of mouth, SOHND: supraomohyoid neck dissection, RFFF: radial forearm free flap, STSG: split thickness skin graft, SND: selective neck dissection, PNI: perineural invasion, ECE: extracapsular extension, LVSI: lymphovascular space invasion, CCRT: concurrent chemoradiotherapy, ALT: anterolateral thigh flap, FFF: fibula free flap, SCM: sternocleidomastoid muscle, IJV: internal jugular vein)
4. Recurrence
Table 6
| Study | Survival outcomes | Surgery-related complications | Radiotherapy-related complications |
Functional outcome |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Recurrence: local, regional, systemic, second primary | DFS, DSS | OS |
Flap survival wound status |
Other | Osteoradionecrosis | Other | ||||
| Deo et al.9 (2013)1 | Local (95%), regional (11.9%), both locoregional (2.4%), systemic (2.8%, in lungs), second primary (7.1% – 2 tongue, 1 nasopharyngeal) | 3-yr, 5-yr DFS 64% | 3-yr OS 71% |
4.8% Oro-cutaneous fistula (2.4%) Flap dehiscence (2.4%) |
- | - | 90% (Not specified) | - | ||
| Huang et al.10 (2001)1,2 | Local (21.5%), regional (10.8%, 76% within 2 yr of Tx), distant (10.8%, 6 lung, 1 bone, 1 liver – 75% within 3 yr of Tx), second primary (32.3%, lung (8), lip (2), pyriform sinus (2), postcricoid (2), hypopharynx (2), nasal cavity (1), tongue base (1), esophagus (1), endometrium (1), breast (1), unknown (1) multiple (2)) |
5-yr DFS 40%: preoperative RT (90%), postoperativeRT (63%), RT alone (31%) (0.0351) T1 (75%), T2 (50%), T3 (72%), T4 (54%, 0.617) N0 (69%), N1 (56%), N2 (26%) (0.0159) |
- | - | - |
7% 6 months – 5.3 years after RT 3 managed conservatively, 2 received hemimandibulectomy, 2 occurred after tooth extraction |
Lower grade 3 bone & soft tissue complication: 10.8% Preoperative RT (0%), postoperativeRT (13%), RT alone (13%) |
- | ||
| Kowalski et al.11 (1993)2,3 | Local (27.2%), regional (1.1%), distant (3.5%), second primary (14.0%, lip (1) oral cavity (5), oropharynx (1), hypopharynx (2), esophagus (3), lung (3), skin (1)) | 5-yr DFS: 48.9% | 5-yr OS: 55.3% | Wound dehiscence (20.1%), wound infection (18.3%), flap necrosis (11.5%), among flap reconstruction (41.9%), fistula (11.5%), carotid rupture (1.8%), hematoma (3.5%) |
51.8% Seroma (7.0%), pneumonia (5.3%), other (2.6%) |
- | - | - | ||
| Petruzzelli et al.13 (2003) | Local (7.7%), regional (0), systemic (7.7%, lung), second primary (7.7%, thoracic esophagus) | DFS: 76.9% | 76.9% | - | - | - | - |
At 1-yr postoperative, with University of Washington Quality of Life Data Only significant problems with chewing (mean QoL score 57.69) |
||
| Binahmed et al.12 (2007)1,3,4,5,6 | Local (26.3%), regional (6.7%), systemic (5.3%), Second primary, (not mentioned) |
5-yr DFS: 40.3% 5-yr DSS: 67.7% |
5-yr OS 51.4% | - | - | - | - | - | ||
| Bayman et al.14 (2010)1,7 | Second primary (14%, lung (3), laryngeal (2), contralateral oropharyngeal (1), previous follicular lymphoma (1)) |
5-yr LRC: 46.5% 5-yr DSS: 45.7% |
5-yr OS 30.9% | - | - |
5.6% All managed conservatively |
Deaths: 9.3% 2 deaths while receiving RT (myocardial infarction (1), bronchopneumonia (1)), 2 deaths of within 3 months RT |
- | ||
| Factor et al.15 (2020)1,8 | Local (29%, 54%), regional (28.6%, 30.8%), systemic (35%, 23%), second primary (not reported) | 2-yr LRC: 68.4% (35.9%), 2-yr DFS: 68.4% (32.7%), median time to failure: 18.5 mo (12.0 mo) | - | - | - | - | - | - | ||
| Ayad et al.16 (2005)1,2,7 | Local (41.3%), regional (10.9%), systemic (lungs, 2.2%), second primary (32.6%, lung (1), new head and neck site (14)) | 2-yr DSS: 84%, 5-yr DSS: 78%, 2-yr LC: 68%, 5-yr LC 49%, ultimate 5-yr LC: 67%, 2-yr & 5-yr RC: 88%, 2-yr LRC: 59%, 5-yr LRC: 42%, ultimate 5-yr LRC: 70% | 2-yr OS: 73%, 5-yr OS: 47% | - | - | 6.5% within 1 year of RT, all managed conservatively |
Death: 0% Severe mucositis leading to significant weight loss & nasogastric tube feeding: 8.7% |
- | ||
| Hitchcock et al.17 (2015)1,2,7 | Locoregional (37%), regional (0%), systemic (11%), second primary (not reported) |
5-yr LC: 64% RT (50%), surgery+RT (71%) 5-yr LRC: 63% RT (50%), surgery+RT (69%) 5-yr distant metastasis-free survival: 89% 5-yr DSS: 62% RT (53%), surgery+RT (67%) |
5-yr OS: 49% RT (39%), surgery+RT (54%) |
Mandibular fracture (3%) Oro-cutaneous fistula (12%), requiring PEG tube (7%) Wound infection requiring hospitalization (8%) Flap failure (1%) |
Surgery+RT (52.7%) RT alone (19.4%) |
10% Definitive RT (5.6%) Surgery+RT (12.2%) (all requiring HBO or surgery) |
Soft tissue necrosis: 3.6% surgery+RT (5%) Bone exposure: 7.3% RT (11%), surgery+RT (5%) Dehydration: 2.7% RT (3%), surgery+RT (3%) Anemia: 0.9% surgery+RT (1) Auditory canal stenosis: 0.9% RT+surgery |
- | ||
| Nishi et al.18 (2018)1,7 | Local (20%), regional (31.1%), systemic (11.1%, lung) | DFS: 44.4%, death (51.1% from original disease, 4.4% from other disease), 3-yr DSS 59.7% |
3-yr OS: 59.8% (T1: 75%, T2: 80%, T3: 40%, T4a: 46.4%) |
- | - | - | - |
Days elapsed from surgery to start of oral intake in flap reconstruction patients (n=38) 1-7 days: 21%, 8-14 days: 55.3%, 15-21 days: 15.8%, ≥22 days: 5.3% Trismus (0), death from postoperative complications (1) |
||
| Byers et al.19 (1984) | Local (13.6%), regional (10.5%), systemic (not reported), second primary (32.7%, aerodigestive tract (18), lung (6), esophagus (5), other (7)) | 5-yr DSS (85.5%) | 5-yr OS: 26% | Oro-cutaneous fistula: 13.3% | - |
26.6% (17/64) 7 managed conservatively, 10 received mandibulectomy |
- | - | ||
| Hao et al.20 (2006)1,3,4,5,9 | Local (18%), regional (8%), systemic (10%), second primary (26%) | - |
5-yr OS (60.6%): I (100%), II (74.1%), III (75%), IV (43.6%) Mean survival time: 71.9 mo Stage I (46.81 mo), II (57.46 mo), III (54.48 mo), IV (62 mo) |
- | - | - | - | - | ||
| Demir and Öztürk Yanaşma21 (2020)1,3,7,10,11 | Locoregional (15%), systemic (20%, lung), second primary (not reported) | (Average 26 mo) DFS (75%) | (Average 26 mo) OS (60%) |
Soft tissue infections resolved by IV antibiotics (15%) Minor re-operations d/t flap dehiscence, necrosis, or scar removal (40%) |
PEG tube placement: 10% | - | - |
Mean time to oral intake and removal of nasogastric feeding: 19.6 days (6-52 days) Mean time to removal of tracheostomy tube: 14 days (2-25 days) |
||
| Rizvi et al.22 (2018)1,2,7,8,9 | - | DSS: 2 yr (68%), 5 yr (55%), 10 yr (45%) | 2 yr (59%), 5 yr (38%), 10 yr (21%) | - | - | - | - | - | ||
| Faisal et al.1 (2017)1 | Local (19.4%), regional (9.6%), systemic (10%), second primary (not reported), persistent disease (24%) | - |
5-yr OS: 38% (22%) 3-yr OS: surgery+RT (76%), RT (56%), CCRT (38%) T1-2 (75%), T3-4 (32%), clear margin (100%), close (63%), involved (23%) |
- | - | - | - | - | ||
5. Survival outcomes
Table 7
| Study | Locoregional, distant control | DFS, DSS | Overall survival | Rate of postoperative complications |
|---|---|---|---|---|
| Deo et al.9 (2013)1 | - | DFS: node status (N0 [92%], N+ [61%]) (P=0.014) | - | - |
| Huang et al.10 (2001)1,2 |
LRC: Tx modality (P=0.046) Distant: N stage (P=0.002) |
DFS: Tx modality (P=0.002), N stage (P=0.012) | - | - |
| Kowalski et al.11 (1993)2,3 | - | DFS: T stage, hard palate involvement, inferior gingiva involvement | T stage, hard palate involvement, FOM involvement | Increase: gingiva (P=0.005), hard palate (P=0.019), use of tongue flap for reconstruction |
| Binahmed et al.12 (2007)1,3,4,5,6 | - | - | Margin status (clear (68%), close (83%), involved (0%), P=0.270) | - |
| Bayman et al.14 (2010)1,7 | - | DSS: none (T stage, node status) | None (T stage, nodal status) | - |
| Factor et al.15 (2020)1,8 | LRC: none (age, sex, stage, margins, ECE, PNI, LVI, CT, tumor site) | - | - | - |
| Ayad et al.16 (2005)1,2,7 |
LC: none, RC: N stage (N0-1>N2-3, P=0.04) Overall stage (I-II>III-IV, P=0.02) |
DSS: T stage (T1-2>T3-4, P=0.02) N stage (N0-1>N2-3, P=0.005) | None (T stage, N stage, overall stage) | - |
| Hitchcock et al.17 (2015)1,2,7 | LC: Tx modality (surgery+RT>RT alone, P=0.0021) LRC: overall stage (P=0.002), Tx modality (P=0.0003) | DSS: overall stage (P=0.01), Tx modality (P=0.0009) |
Overall stage (P=0.01), Tx modality (P=0.004) No impact: T stage, N stage, sex, ethnicity |
- |
| Nishi et al.18 (2018)1,7 | - | - |
Clinical node status (P=0.0222) Histopathological node status (P=0.0001) |
- |
| Byers et al.19 (1984) |
LC: T stage (T1: 92%, T2: 88%, T3: 90%, T4: 75%), Tx modality (surgery alone): 89.1% (without mandibulectomy: 92%, mandibulectomy: 87.5%), RT alone: 84%, (preoperative: 0%, postoperative: 18.2%) RC: N stage (N0: 89%, N1: 86%, N2: 83%, N3: 66%) neck dissection (RND: 83%, mRND: 90%, SOHND: 94%) |
- | - | - |
| Hao et al.20 (2006)1,3,4,5,9 | Local recurrence: bone invasion (P=0.047), second primary (P=0.04) | - | Overall stage (P=0.049), node status (P=0.04) masticatory space invasion (P=0.01), neck recurrence (P=0.04) | - |
| Demir and Öztürk Yanaşma21 (2020)1,3,7,10,11 | - | Multilevel lymph node metastasis (P=0.013) (No impact: T stage, PNI, tumor grade—The authors attributed this to the small study population.) | - | - |
| Rizvi et al.22 (2018)1,2,7,8,9 | - | DSS: black race (HR: 1.46, P=0.002), age (HR: 1.01), surgery (HR: 0.46), RT, tumor size, N stage, overall stage (HR: 1.57), size (1.002, 2-4 cm [HR: 1.76], >4 cm [HR: 3.14]), all except race: P<0.001 | Age, surgery, RT, tumor size, N stage, overall stage (all P<0.001) | - |
| Faisal et al.1 (2017)1 | - | - |
Tx modality (surgery+RT [76%]>RT only [56%]>CCRT [38%]) Overall stage (I-II [75%]>III-IV [32%]) Margin status (clear [100%]>close [63%]>involved [23%]) P value not stated, not statistically analyzed. |
- |
(DFS: disease-free survival, DSS: disease-specific survival, LRC: locoregional control, Tx: treatment, FOM: floor of mouth, ECE: extracapsular extension, PNI: perineural invasion, LVI: lymphovascular space invasion, CT: chemotherapy, LC: local control, RC: regional control, RT: radiotherapy, RND: radical neck dissection, mRND: modified radical neck dissection, SOHND: supraomohyoid neck dissection, HR: hazard ratio)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download