I. Introduction
Wound healing is an integral part of oral surgery, and it plays an important role in patient prognosis. After any surgical procedure in the oral cavity, it takes a minimum of one to one-and-a-half months for the wound to heal completely
1.
Appropriate wound closure is of great importance in any surgical procedure, especially intraoral procedures
2,3. Healing can occur in two ways: primary intention healing and secondary intention healing. Healing that occurs without scar tissue formation is termed primary intention and healing that occurs with granulation tissue formation is termed secondary intention
1,2.
Primary healing occurs in clean and neat wounds with no infection, while wounds with uneven edges and infections generally heal through secondary intention
1. Factors such as bleeding, vascularity, granulation tissue formation, suppuration, tissue discoloration, and pain are determining factors of wound healing
4.
Various factors aid in wound healing and numerous materials and techniques are continuously proposed to aid in wound healing, which can be classified into pharmacological agents and non-pharmacological agents. The pharmacological agents include various drugs that aid in healing like anti-inflammatory drugs, immune-suppressants, and antimicrobial drugs.
Bioinert materials and membranes include plasma rich fibrin (PRF), plasma rich platelets (PRP), bone grafts, skin grafts, collagen membrane, and hydrocolloid materials
2,3. The disadvantage of these materials is that they require a separate surgical site or a donor site. The patient must undergo secondary procedures for graft procurement. Collagen membrane has challenging handling properties. PRF and PRP require a blood draw from patients. Thus, there is a need for materials that have easy handling properties, are inexpensive, and can be made easily available to patients. One such material is amniotic membrane.
The amniotic membrane is the innermost layer of the placenta, consisting of a thick basement membrane and an avascular stromal matrix
1,4. It can be used as a graft and as a dressing to facilitate reconstruction and to promote healing
4. It is an emerging novel method that aids in wound healing
4,5. It is widely used for better healing in diabetic foot, as a burn dressing, and for orbital surgery. The amniotic membrane is an excellent graft material that helps heal soft and hard tissue.
Certain surgical procedures are only carried out in the oral cavity. Alveoloplasty is often performed prior to receiving a denture or to bring pain relief. The main procedural objective of alveoloplasty is to round out sharp bony edges and remove any bony spicules or undercuts present after an extraction
5,6. The amniotic membrane has been used in the oral cavity for pre-cancerous lesions and procedures like vestibuloplasty and is also commonly used with alveoloplasty. However, a thorough search of the English-language medical literature revealed no evaluations of amniotic membrane for wound healing after alveoloplasty. This pilot study aims to assess amniotic membrane efficacy for wound healing after alveoloplasty.
Go to :

II. Materials and Methods
This prospective split-mouth pilot study was conducted in the Department of Oral & Maxillofacial Surgery, Dr. D. Y. Patil Vidyapeeth after an ethical clearance was granted by the Institutional Review Board (No. DPU/1185/19). Using a convenience sampling technique with random allocation, 10 patients with bilaterally edentulous jaws who reported to our center and required bilateral alveoloplasty procedure were selected.
Sample size: n=20 alveoloplasty sites; n=[(Z1-α–Zβ)6]2/3
Site A: n=10 (with dehydrated human amniotic/chorionic membrane [dHACM])
Site B: n=10 (without dHACM)
Patients who fit the following inclusion criteria were chosen for the study: (i) indicated for alveoloplasty in the upper and lower jaw bilaterally; (ii) >45 years or older; and (iii) willing to participate. The exclusion criteria were: (i) history of tobacco chewing, smoking, or alcohol consumption and (ii) any of the following medical conditions—coagulation disorders, metabolic bone disorders, drug consumption leading to gingival hyperplasia, pregnancy, history of radiotherapy, chemotherapy, or having recently undergone any operative procedures.
A detailed history was taken of all enrolled participants. All participants were well-informed of the study goals and procedures and everyone was given a printed informed consent form. All necessary preoperative radiographs that is—Orthopantomagram has been taken. Preoperative workups, such as a complete blood profile, were performed for all patients. Site selection and dHACM placement side (TATA Tissue Bank, Mumbai, India;
Fig. 1) were determined using the Sequentially Numbered Opaque Sealed Envelopes (SNOSE) technique.
 | Fig. 1Dehydrated human amniotic/chorionic membrane obtained from human placental extract. 
|
One operator carried out all alveoloplasty surgical procedures and another investigator recorded the healing index at seven days and 21 days postoperatively using the Landry, Turnbull, and Howley Index.(
Table 1)
Table 1
Landry, Turnbull, and Howley Index for checking the healing of soft tissues
|
Healing index |
Tissue color |
Bleeding palpation |
Granulation tissue |
Incision margins |
Suppuration |
|
1 (very poor: Two or more signs are present.) |
>50% of red gingiva |
Yes |
Yes |
Not epithelialized, with loss of epithelium beyond incision margin. |
Yes |
|
2 (poor) |
>50% of red gingiva |
Yes |
Yes |
Not epithelialized, with exposed connective tissue. |
No |
|
3 (good) |
25%-50% of red gingiva |
No |
No |
No exposed connective tissue. |
No |
|
4 (very good) |
>25% of red gingiva |
No |
No |
No exposed connective tissue. |
No |
|
5 (excellent) |
All pink tissues |
No |
No |
No exposed connective tissue. |
No |

Patients were taken for surgery and routine painting and draping were performed. Local anesthetic injection of 2% lignocaine hydrochloride with 1:200,000 adrenaline was administered at the alveoloplasty region. A crestal incision was made and a full-thickness mucoperiosteal flap was raised using a Molts Periosteal Elevator (No. 9; iM3, St., Vancouver, WA, USA). Any irregular bone was levelled and smoothened with a bone rongeur and bone file.(
Fig. 2) For all 10 patients, the amniotic membrane was placed at site A and was not placed in site B.(
Fig. 3) Wound closure was done with 3-0 silk sutures.(
Fig. 4) Gauze was placed after surgery. The same procedure was repeated at site B. Postoperative instructions were given to the patient. Analgesics (Paracip 500 mg; Cipla GX, Mumbai, India) were prescribed. The patient was called the next day for follow-up and again on the seventh and 21st days. Healing was measured using the Landry, Turnbull, and Howley Index. The analysis was performed using IBM SPSS Statistics software (ver. 22.0; IBM, Armonk, NY, USA). A
P<0.05 was considered statistically significant.
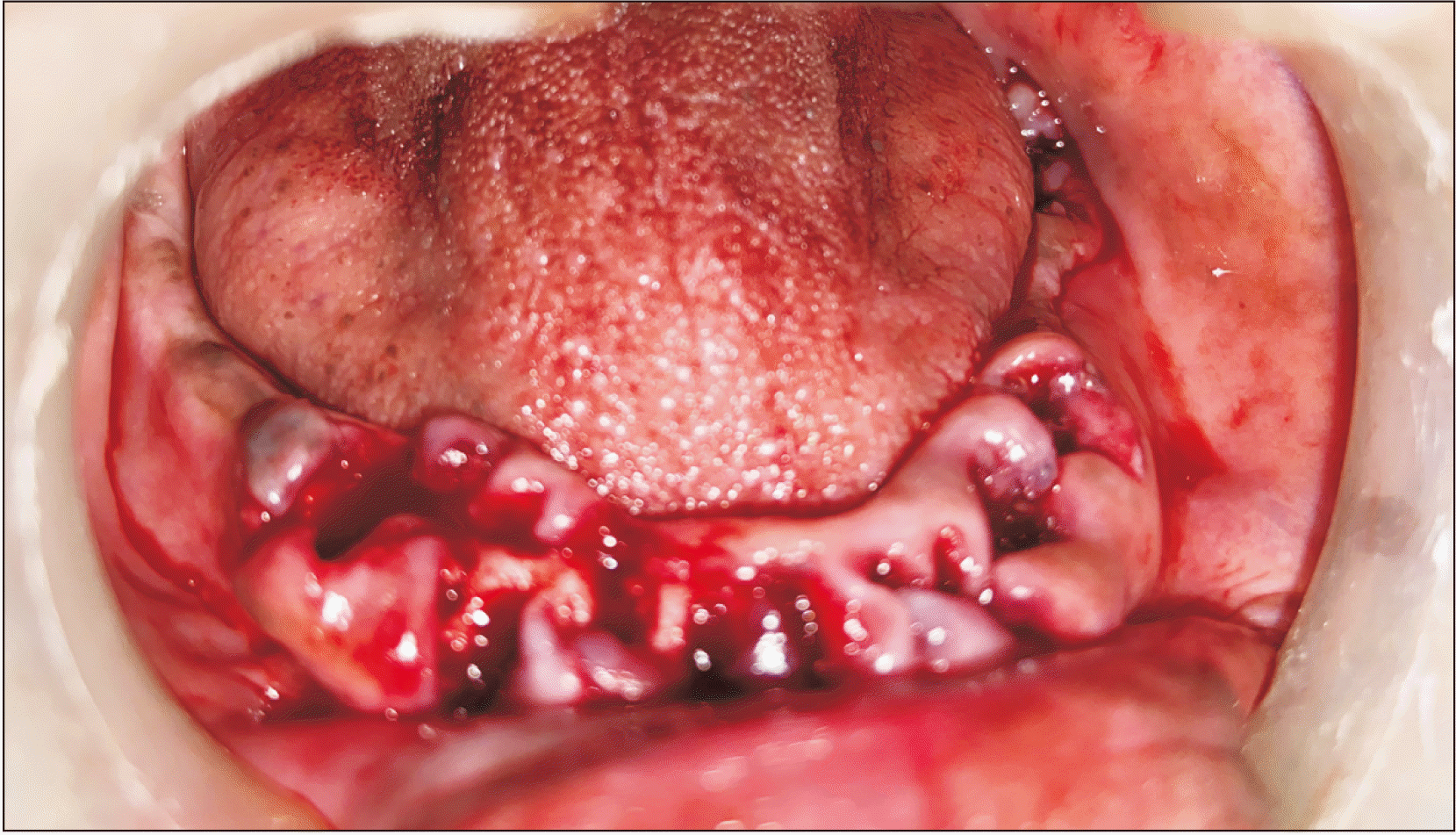 | Fig. 2Alveoloplasty procedure done on lower jaw. 
|
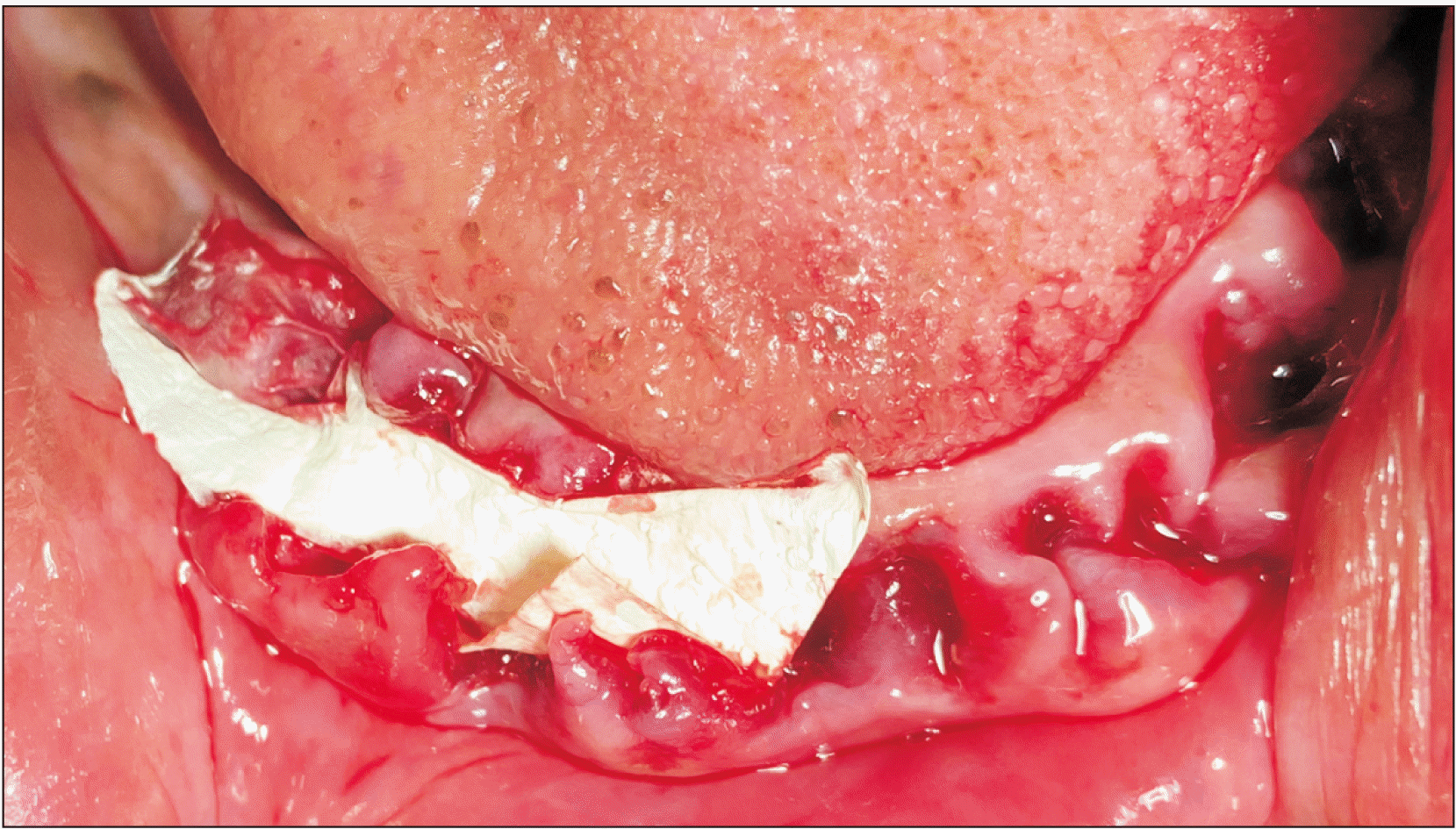 | Fig. 3Dehydrated human amniotic/chorionic placed after alveoloplasty procedure. 
|
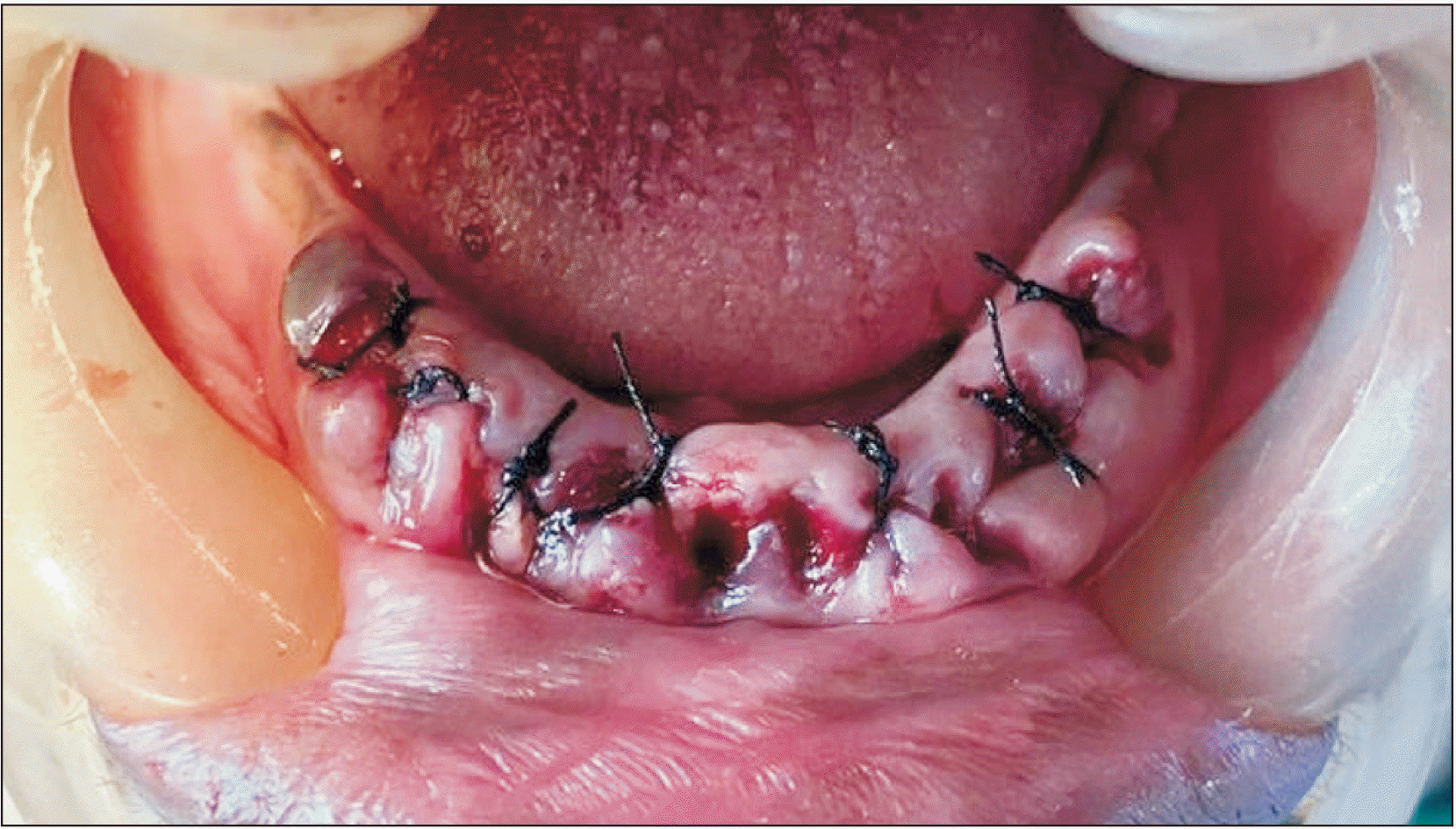 | Fig. 4Closure done with 3-0 silk after placement of dehydrated human amniotic/chprionic procedure. 
|
Go to :

III. Results
This experimental split-mouth study was conducted from 2019 to 2020 to assess the efficacy of dHACM for accelerating the wound healing process. Most patients were between 45-55 years with a mean age of 50.5±10.50 years. Of 10 patients, 5 were male and 5 were female.(
Fig. 5)
 | Fig. 5Demographic chart of patients. 
|
Wound healing was compared between the two sites using the Landry, Turnbull, and Howley Index. A score of 5 indicated excellent tissue healing while a score of 1 indicated very poor healing. Among site A (dHACM) cases, scores ranged from 2 to 5, while site B (no dHACM) cases ranged from 1 to 5. Though the individual parameters that make up the index were not significantly different between the two site groups, the total count, i.e., the healing index, was different.(
Fig. 6) Tissue color comparisons did not reveal significant differences between the two groups (
Fig. 7;
P>0.05. The inter-group comparison of the healing index, which consists of tissue color, bleeding on palpation, granulation tissue, suppuration, and overall healing, was significantly different (
P<0.05, Mann–Whitney U test value=22;
Fig. 8).
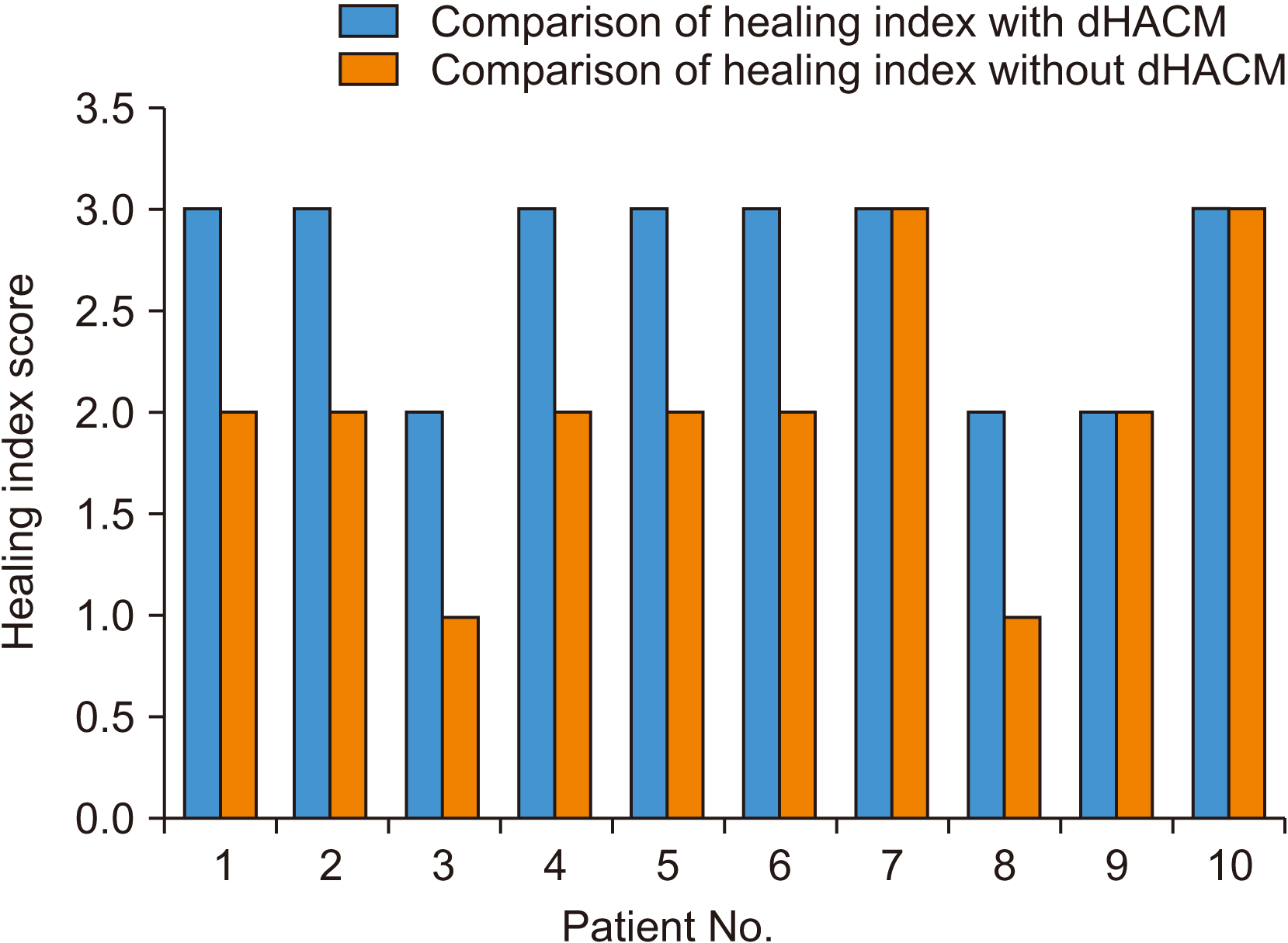 | Fig. 6Comparison of healing index. Mann–Whitney U test value=22, P<0.05. There is a significant difference between the two groups. (dHACM: dehydrated human amniotic/chorionic membrane) 
|
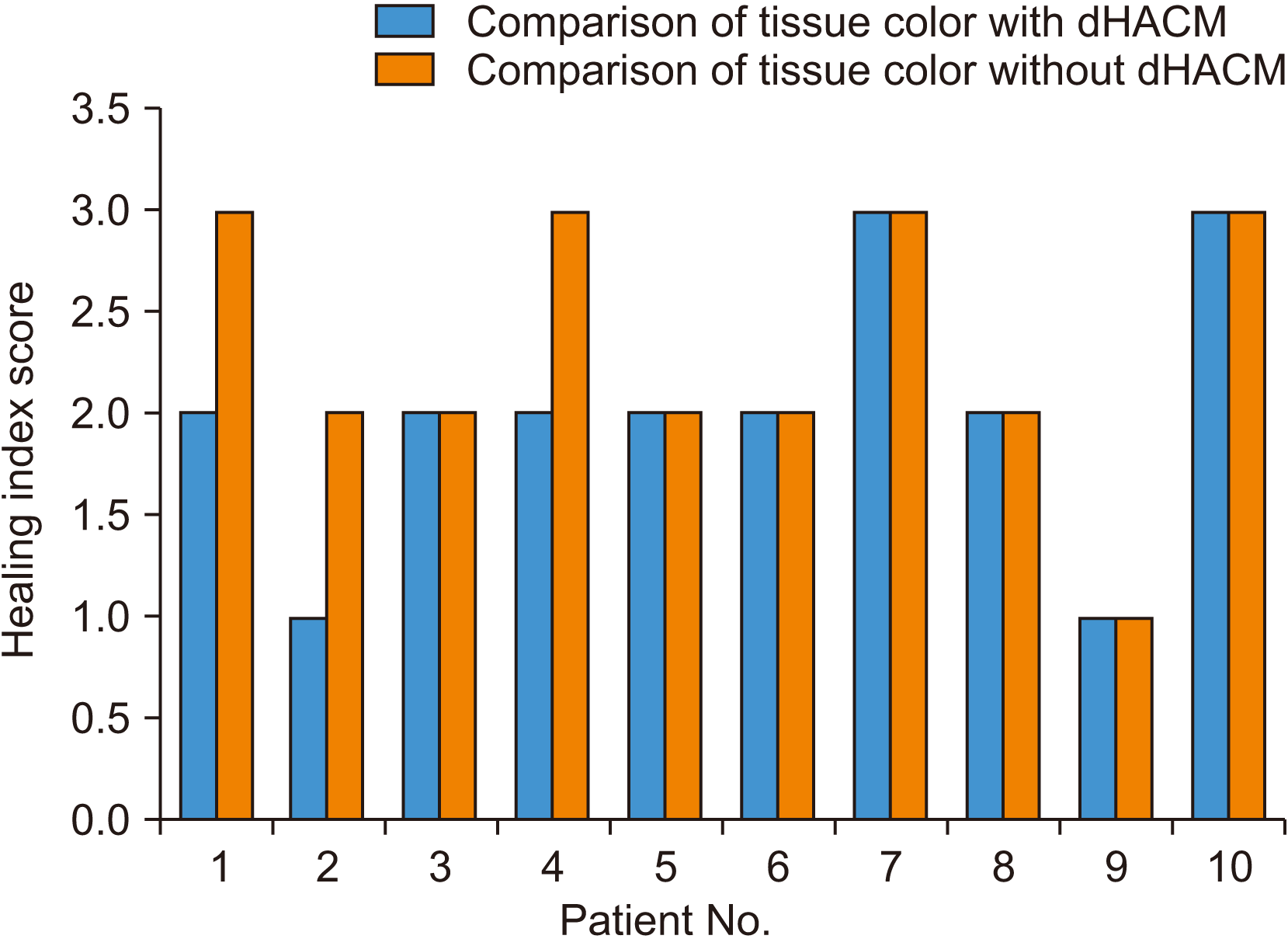 | Fig. 7Comparison of tissue color. Mann–Whitney U test value=38, P>0.05. There is no statistically significant difference between the two groups. (dHACM: dehydrated human amniotic/chorionic membrane) 
|
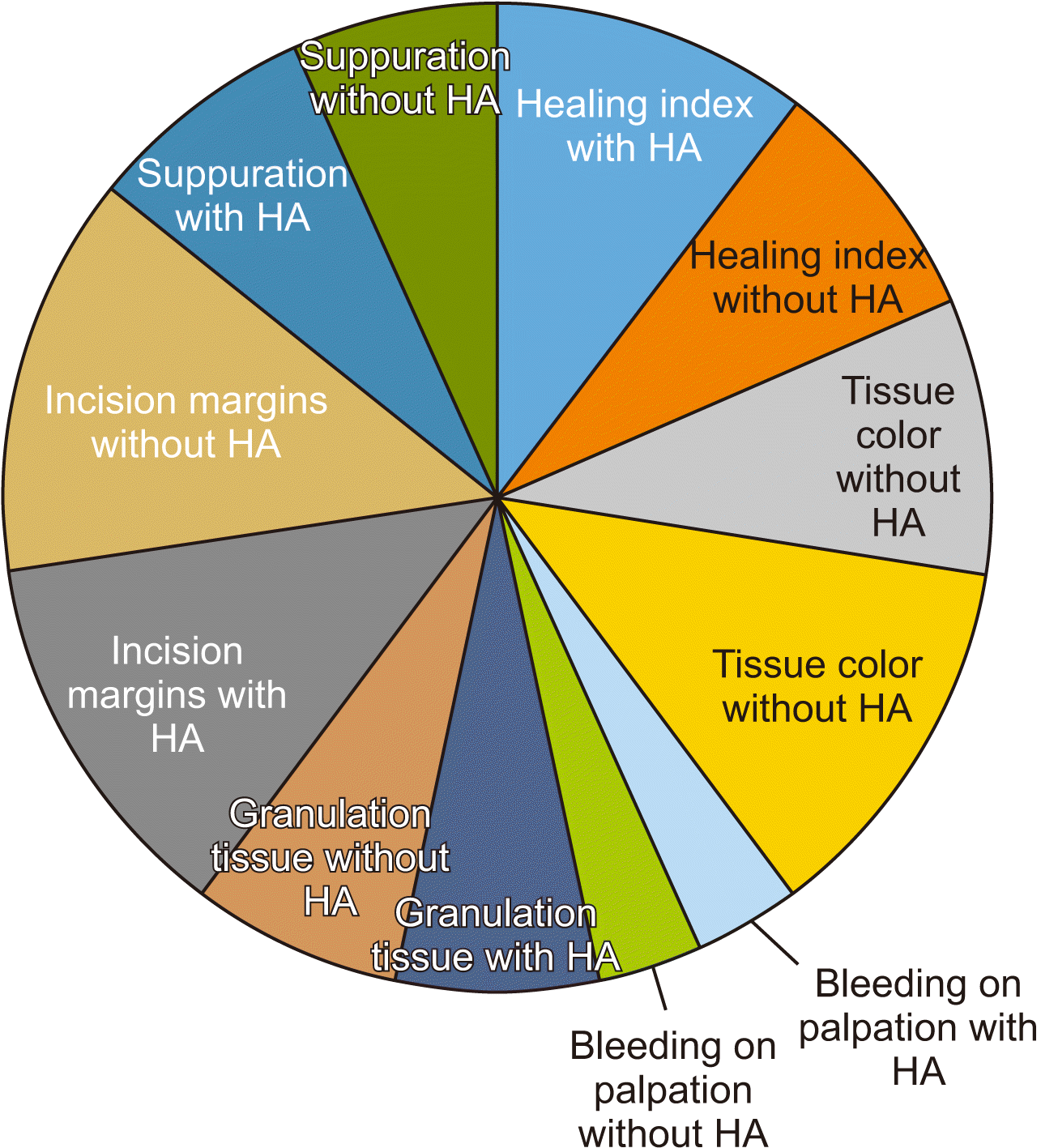 | Fig. 8Overall comparison of healing index of soft tissues. (HA: dehydrated human amniotic/chorionic membrane). 
|
Go to :

IV. Discussion
Denture prosthetics are important for restoring quality of life to edentulous patients. Loss of teeth leads to loss of facial contour, low self-esteem, and, ultimately, compromised aesthetics
5. A primary requisite for receiving good dentures is a smooth alveolar ridge. Traumatic extraction and poor healing of extraction sockets often lead to bony spicules on the alveolar ridge, which makes denture prosthesis difficult and even painful
6.
Furthermore, appropriate wound closure after any intraoral surgical procedure is always of great concern
7. After alveoloplasty, good healing is important for successful receipt of the fabricated denture. In 2016, Politis et al.
1 conducted a study on wound healing in the oral cavity, in which they defined the mouth as an enigmatic structure in which wound healing occurs amidst millions of bacteria. Healing of hard tissue requires approximately six months
1. Various materials and grafts can be used as adjuncts or catalysts for wound healing, particularly PRF, PRP, skin grafts, and dHACM
8.
The amnion is the deepest layer of the human placenta
8-19, and it has distinctive properties. It is made up of three lamina: an overlying tissue, a basement sheath, and a stromal layer beneath
13,14. It possesses the unique property of low immunogenicity, which makes it an excellent grafting material. It behaves as a barrier and does not allow for fibrous tissue proliferation
12,15. The amnion also possesses antiangiogenic and anti-inflammatory substances, including interleukin (IL)-1 receptor antagonist, tissue inhibitors of metalloproteinase-1, -2, -3, and -4, and IL-10.31 and -32
15-17.
Because of these characteristics, dHACM is thought to foster new blood vessel growth, encourage tissue proliferation, and recruit mesenchymal progenitor cells that ultimately aid in better wound healing
17. Its ease of use and obtainment has led to widespread use in many medical areas
17,18.
According to Zhou et al.
20, the amniotic membrane also possesses osteoblastic properties that encourage new bone formation. Similar effects were observed in our previous study when dHACM was used as a graft material for soft tissue healing, and it also contributed to uneventful healing of the alveolar bone underneath
21-23. In this study, a comparison of wound healing efficacy with and without dHACM was performed in alveoloplasty patients. The parameters assessed were the healing index, tissue color, bleeding on palpation, granulation tissue, and suppuration
18.
This study compared bilateral sites in the same patients. This approach offers the unique advantage of reducing variables like immune response, healing capacity, and inflammatory reaction. Because both sites are in the same patient, all these factors are controlled for.
Doig and Simpson
24 carried out an experiment that randomized patients into various groups according to medications and discussed the various randomization strategies to conceal randomization arrangements. They found that the SNOSE strategy was both powerful and affordable. This technique was used in this study to reduce bias
21.
There are many parameters to assess wound healing, but the Landry, Turnbull, and Howley Index is easy and convenient to score
4. It has a scoring range from 1 to 5, the lowest being 1 and the highest being 5. The parameters measured in this index are the healing index, tissue color, bleeding on palpation, granulation tissue, and suppuration, and this index was selected to compare wound healing in alveoloplasty patients with and without dHACM
4.
Although no significant variations were found in any of the Landry, Turnbull, and Howley Index parameters, the mean overall healing index score was higher in sites where amniotic membrane was used. In seven patients, the healing index was higher on the amniotic membrane side than the non-membrane side; the other three patients showed similar healing in both sites. This can be attributed to the low level immune response of the amniotic membrane
15-17,19. According to a 2020 study by Puyana et al.
21, amniotic tissue cells expressed microbial peptides like IL-10, tumor necrosis factor (TNF)-α, and interferons, which are known to inhibit inflammation. In this study, a similar effect was seen because dHACM is anti-inflammatory by nature. The results were significantly different between the sites, so it can be concluded that dHACM is better than suturing alone.
The other parameter assessed was tissue color. According to a 2014 study by Tsuno et al.
16, the amniotic membrane enhances formation of new blood vessels. Identical effects were found in our study, which yielded similar results following new angiogenesis, resulting in improved tissue color. Although tissue color was not significantly different between the two site groups, six out of 10 patients showed better tissue color on their dHACM-treated side than their suture-alone side.
dHACM is a promising material for wound healing. Many studies have concluded that is has multiple effective uses in many medical areas. The advantages of dHACM include its easy handling properties, convenient procurement process, cost efficiency, no requirement of a separate surgical site, and no fear of immuno-histocompatibility rejection
11,12,15,16,18. No studies have reported any adverse reactions caused by dHACM.
This is a pilot study with a small sample size. A multi-center study with a larger sample size is needed to corroborate our study results.
This study was conducted in older patients, where healing is delayed compared with younger patients. The study shows that amniotic membrane enhances healing in this population.
Go to :


