I. Introduction
II. Materials and Methods
1. Preparation of demineralized tooth bone (dTB)
2. Surgical procedures
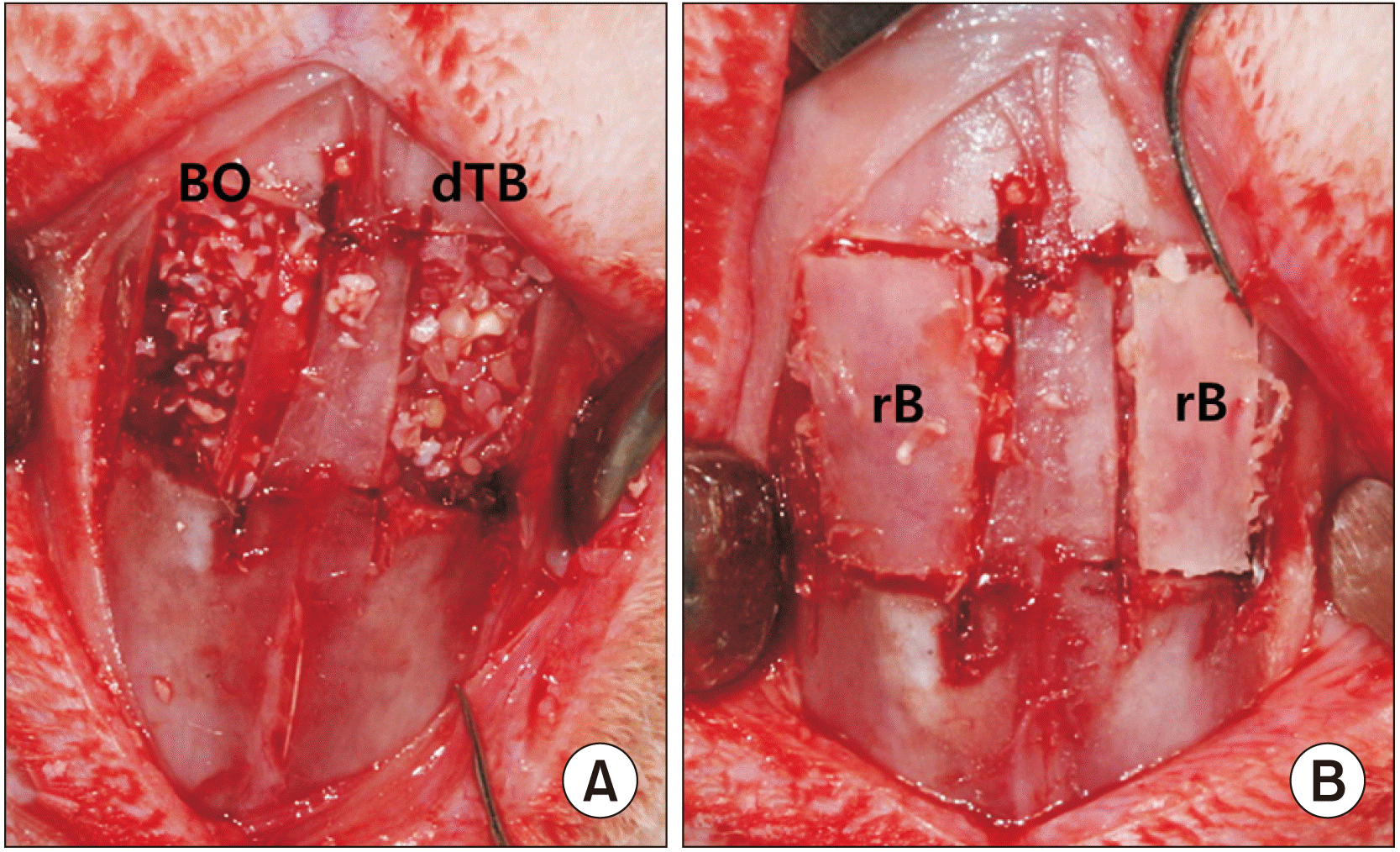 | Fig. 1In the control group, Bio-Oss material was grafted into the new compartments established under the elevated sinus membrane on the left side. In the experimental group, demineralized particulate human tooth bone was grafted into the same area on the right side (A). In both groups, the replaceable bony window was replaced over the bone graft (B). (BO: Bio-Oss, dTB: demineralized particulate human tooth bone, rB: replaceable bone) |
3. Tissue preparation
4. Bromodeoxyuridine (BrdU) Immunohistochemistry
5. Number of BrdU-labeled cells
6. Immunohistochemical assay
III. Results
1. Histological analysis
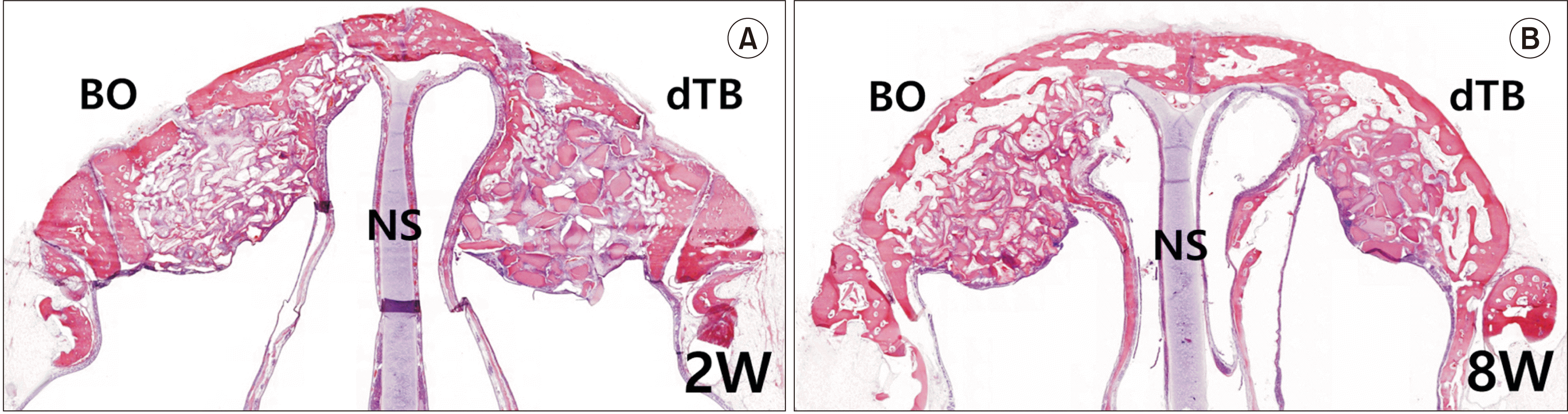 | Fig. 2Coronal sections of a rabbit nasal cavity stained with H&E (×12.5) at two weeks (2W) (A) and eight weeks (8W) (B) after surgery. In each image, the left side shows the grafted sinus treated with Bio-Oss material after maxillary sinus membrane elevation with replacement of the bony window, while the right side shows the grafted sinus treated with demineralized particulate human tooth bone with replacement of the bony window. (BO: Bio-Oss, dTB: demineralized particulate human tooth bone, NS: nasal septum) |
2. BrdU immunohistochemistry
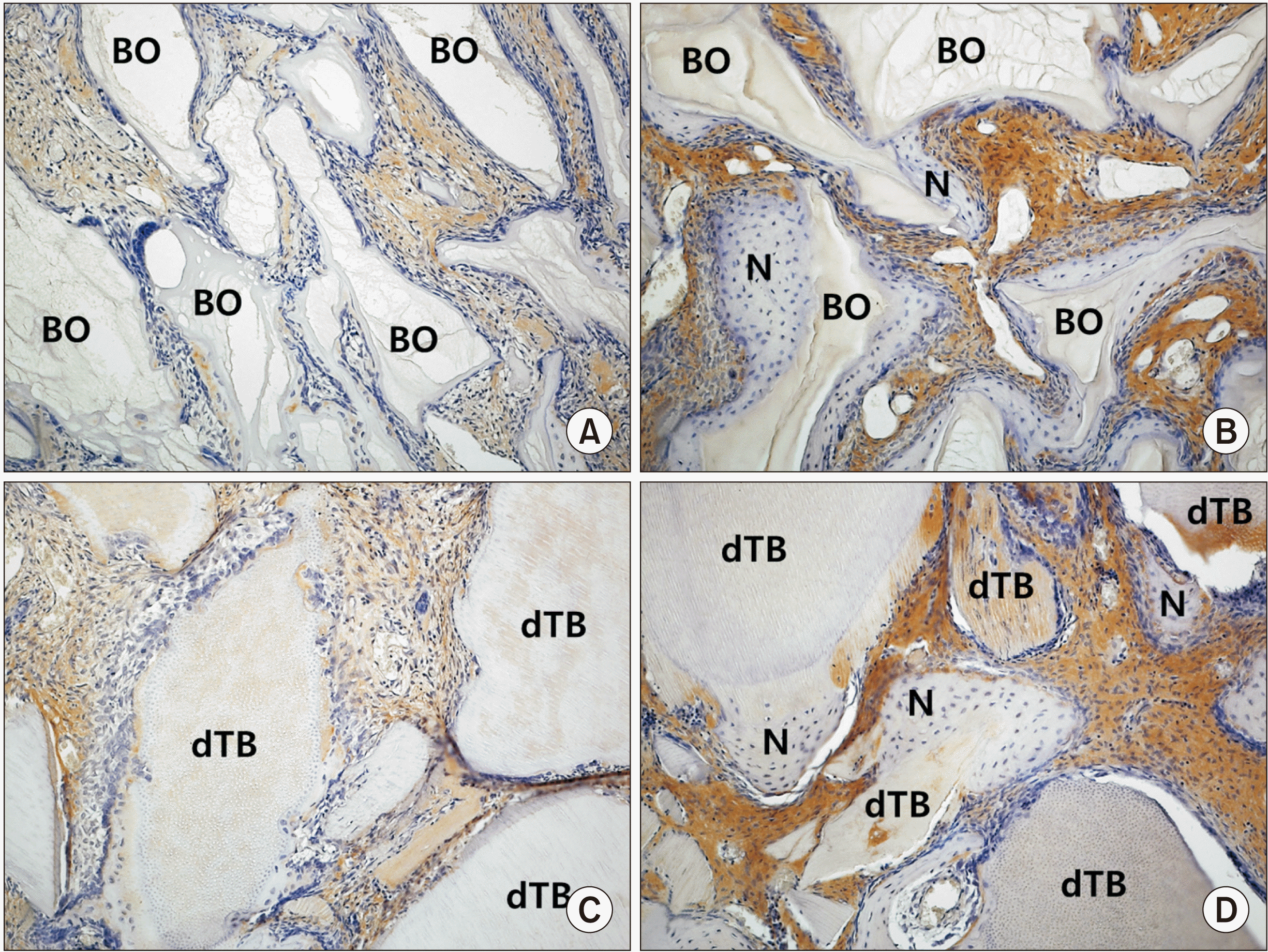
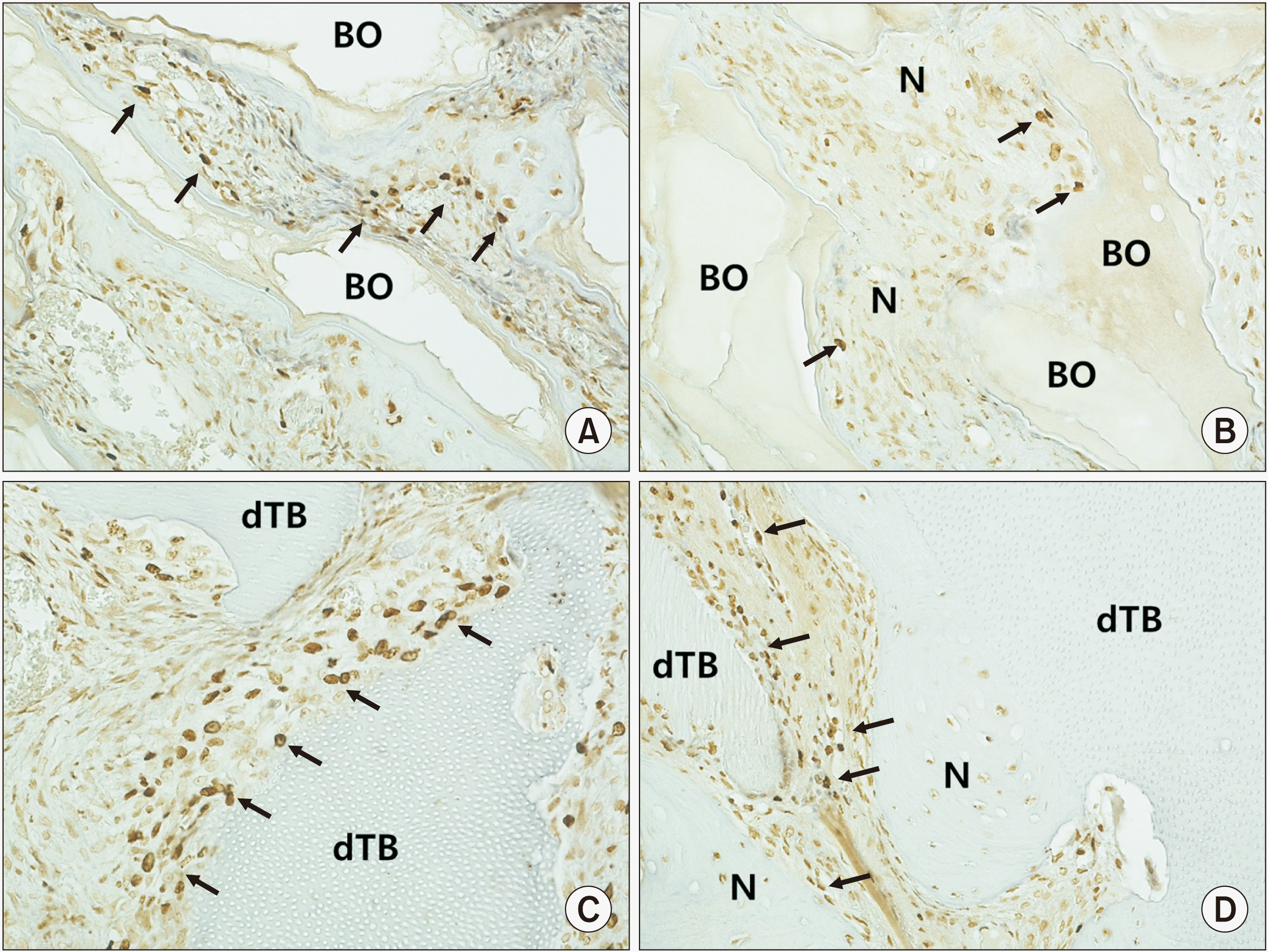 | Fig. 3Immunohistochemical staining (×200) for bromodeoxyuridine (BrdU) in the control group at two weeks (A) and eight weeks (B) and the experimental group at two weeks (C) and eight weeks (D). BrdU-positive cells (arrows) confirmed the existence of osteoblasts on the surface of newly formed bone and graft materials. (BO: Bio-Oss, N: newly formed bone, dTB: demineralized particulate human tooth bone) |
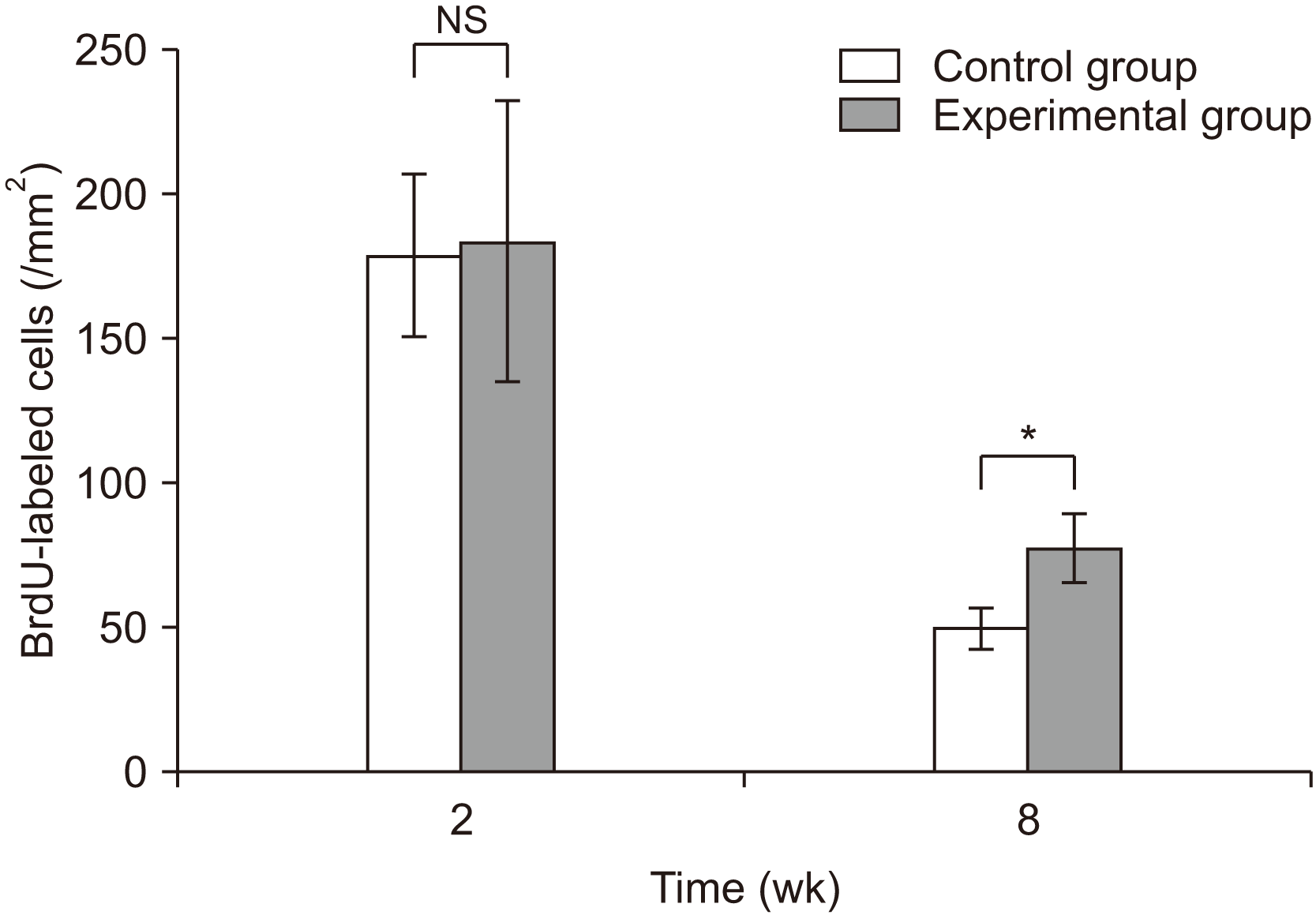
3. Number of BrdU-labeled cells
4. Expression of PCNA
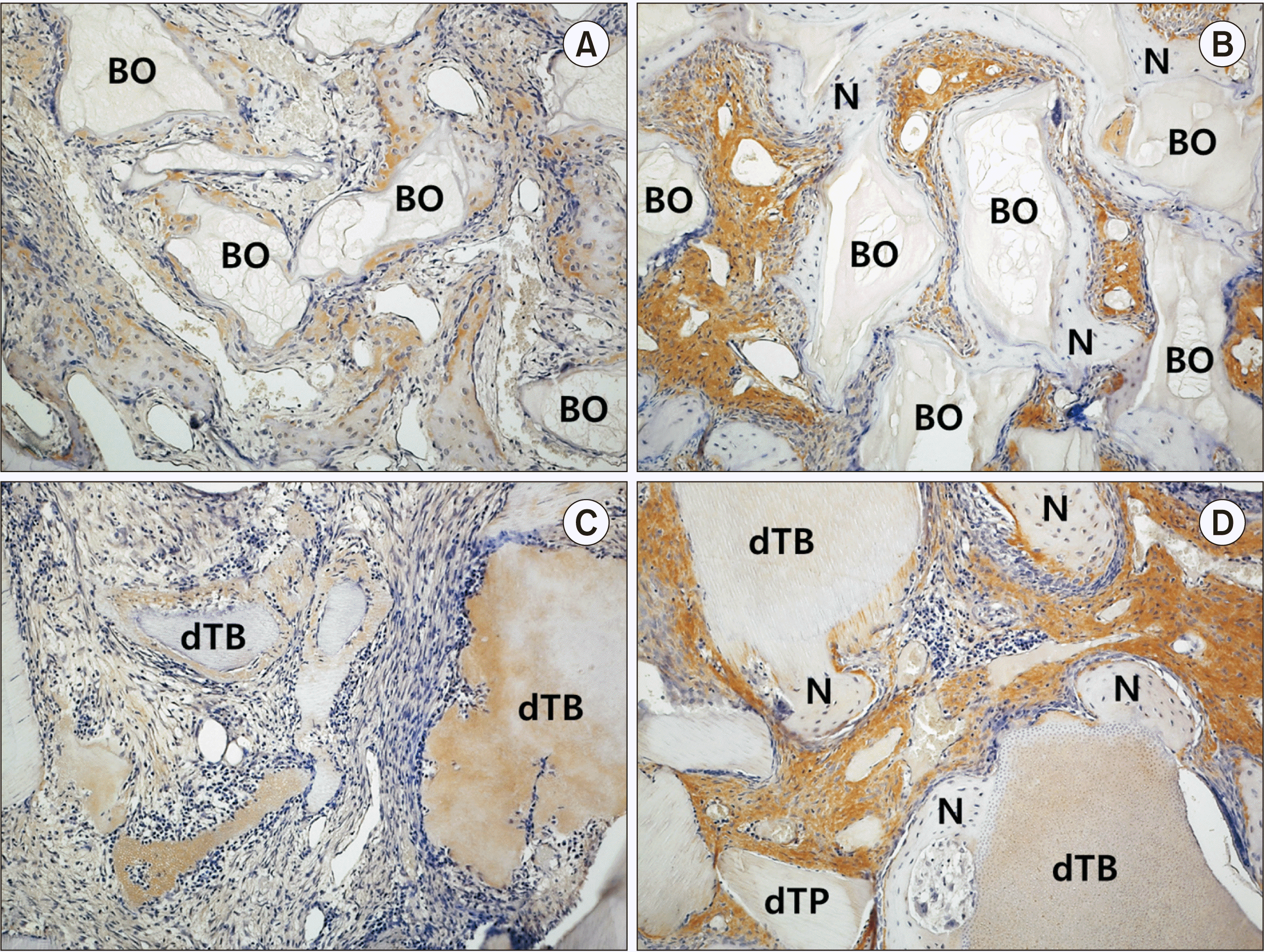
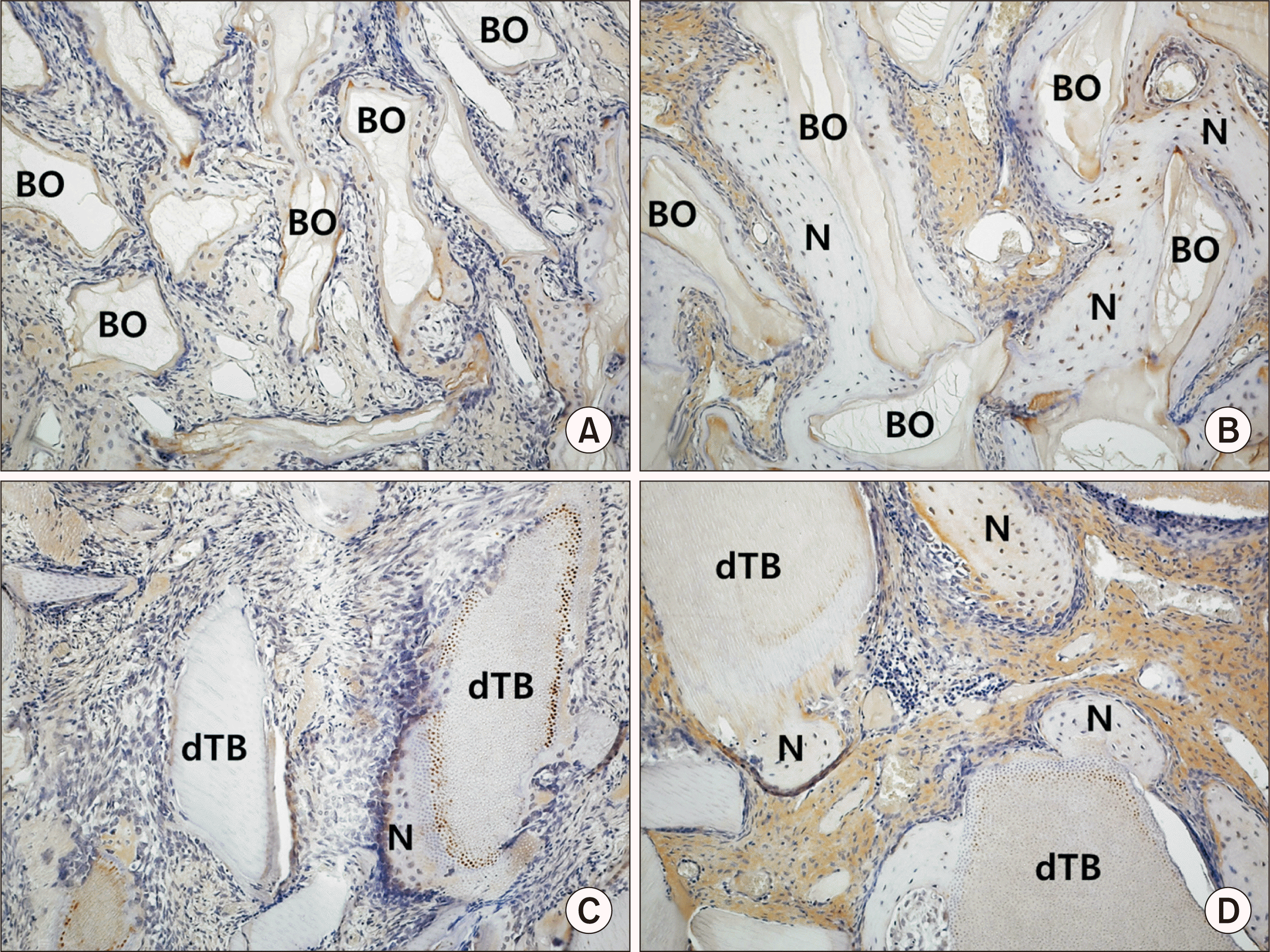
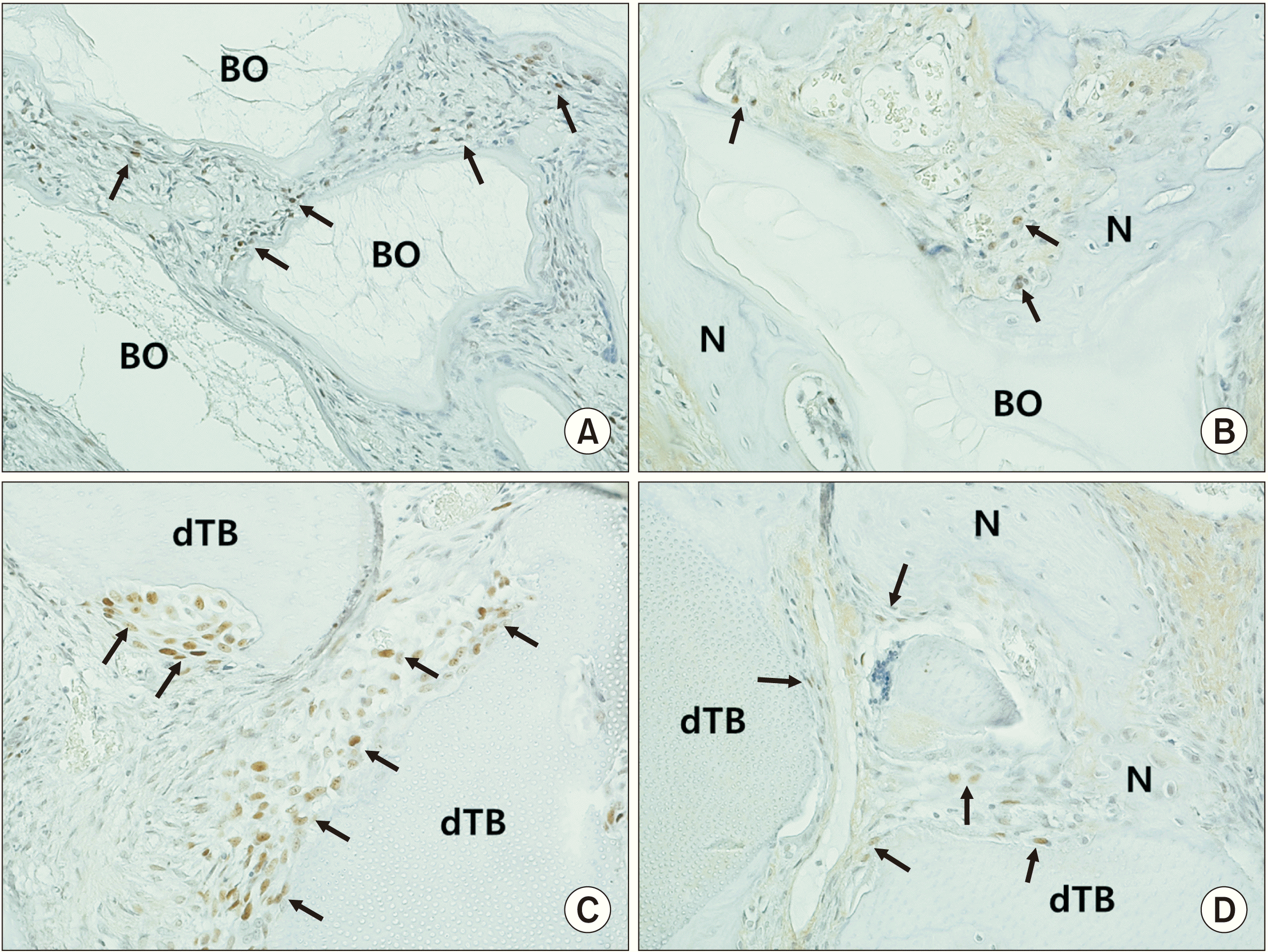 | Fig. 5Immunohistochemical staining (×200) with proliferating-cell nuclear antigen (PCNA) in the control group at two weeks (A) and eight weeks (B) and the experimental group at two weeks (C) and eight weeks (D). Arrows indicate PCNA-positive cells. (BO: Bio-Oss, N: newly formed bone, dTB: demineralized particulate human tooth bone) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download