Abstract
Acinar cell cystadenoma, also known as an acinar cystic transformation of the pancreas, is an exceedingly rare but benign pancreatic lesion. A 51-year-old woman was transferred to Inje University Busan Paik Hospital because of an 8 cm-sized calcified, multiseptated, and multilocular cystic mass in the pancreatic tail observed during abdominal CT performed at another hospital. The patient did not complain of abdominal pain or other symptoms, and her laboratory findings were normal. MRI showed that the cyst was not connected to the main pancreatic duct. A pancreatic serous cystadenoma was suspected, and a laparoscopic distal pancreatectomy was performed. The resected mass was composed of variable sized multilocular cysts with incomplete septa and focally lined by epithelium with acinar differentiation. The patient was diagnosed with acinar cell cystadenoma and is currently being followed up regularly. No complications or recurrences have been observed.
Go to : 
Acinar cell neoplasms of the pancreas are quite rare and include acinar cell carcinoma, acinar cell cystadenocarcinoma, and acinar cell cystadenoma.1 Among them, acinar cell cystadenoma, also known as an acinar cystic transformation of the pancreas, is a cystic lesion of the pancreas that is very uncommon, benign, and shows evidence of acinar differentiation.1,2 This paper reports a case of acinar cell cystadenoma, which was misrecognized as a serous cystadenoma of the pancreas.
Go to : 
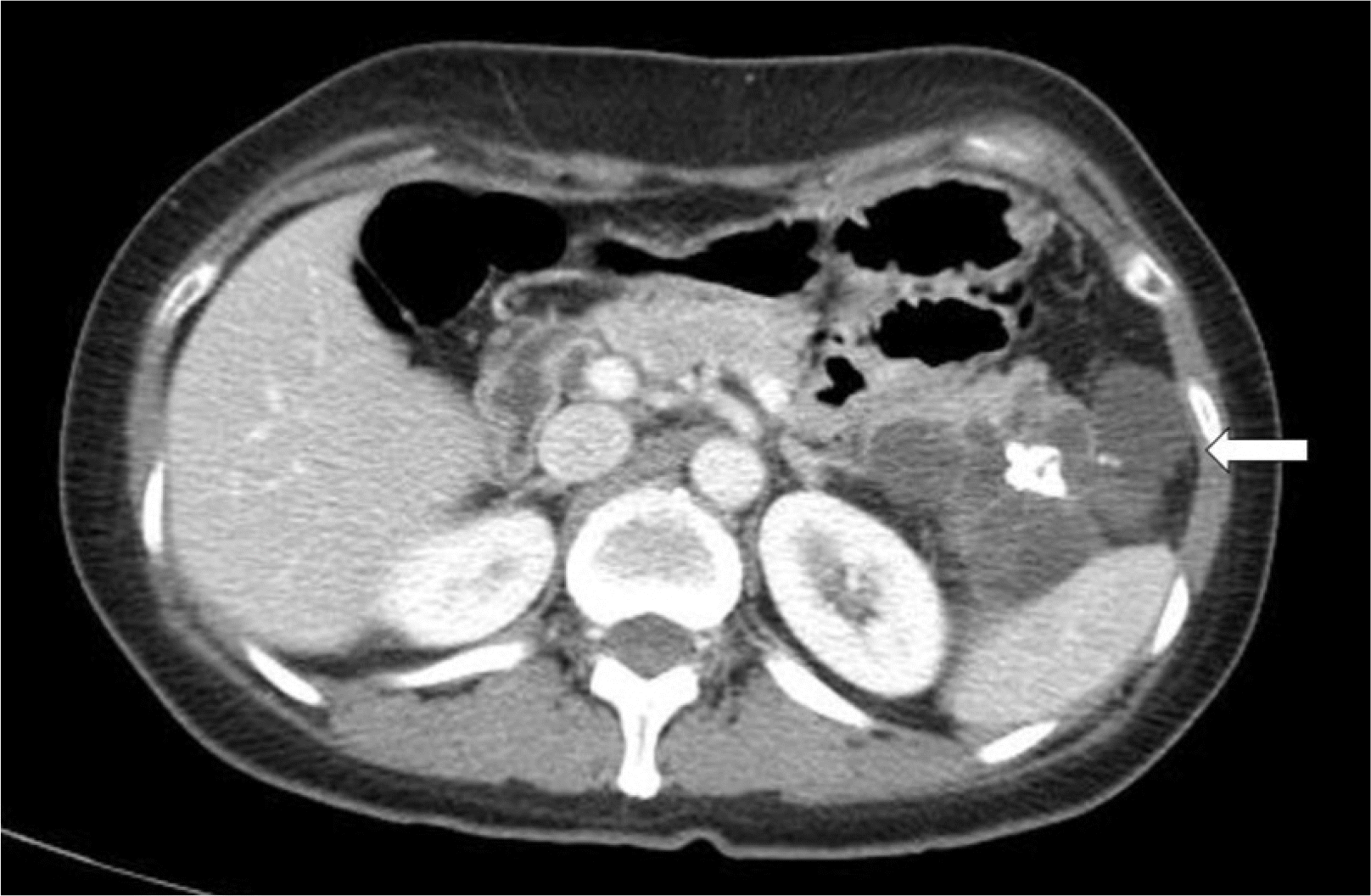
A 51-year-old woman with no significant medical history other than dyslipidemia was transferred to gastroenterology of Inje University Busan Paik Hospital after an 8 cm-sized calcified, multiseptated, and multilocular cystic mass was observed in the pancreatic tail on a CT scan performed in another hospital (Fig. 1).
There were no abdominal symptoms, such as pain, and the tumor marker test was negative at CEA 0.904 ng/mL (reference value ≤4.7 ng/mL), CA 19-9 12.8 U/mL (reference value ≤34 U/mL). The patient was neither a drinker nor a smoker and had no significant family history.
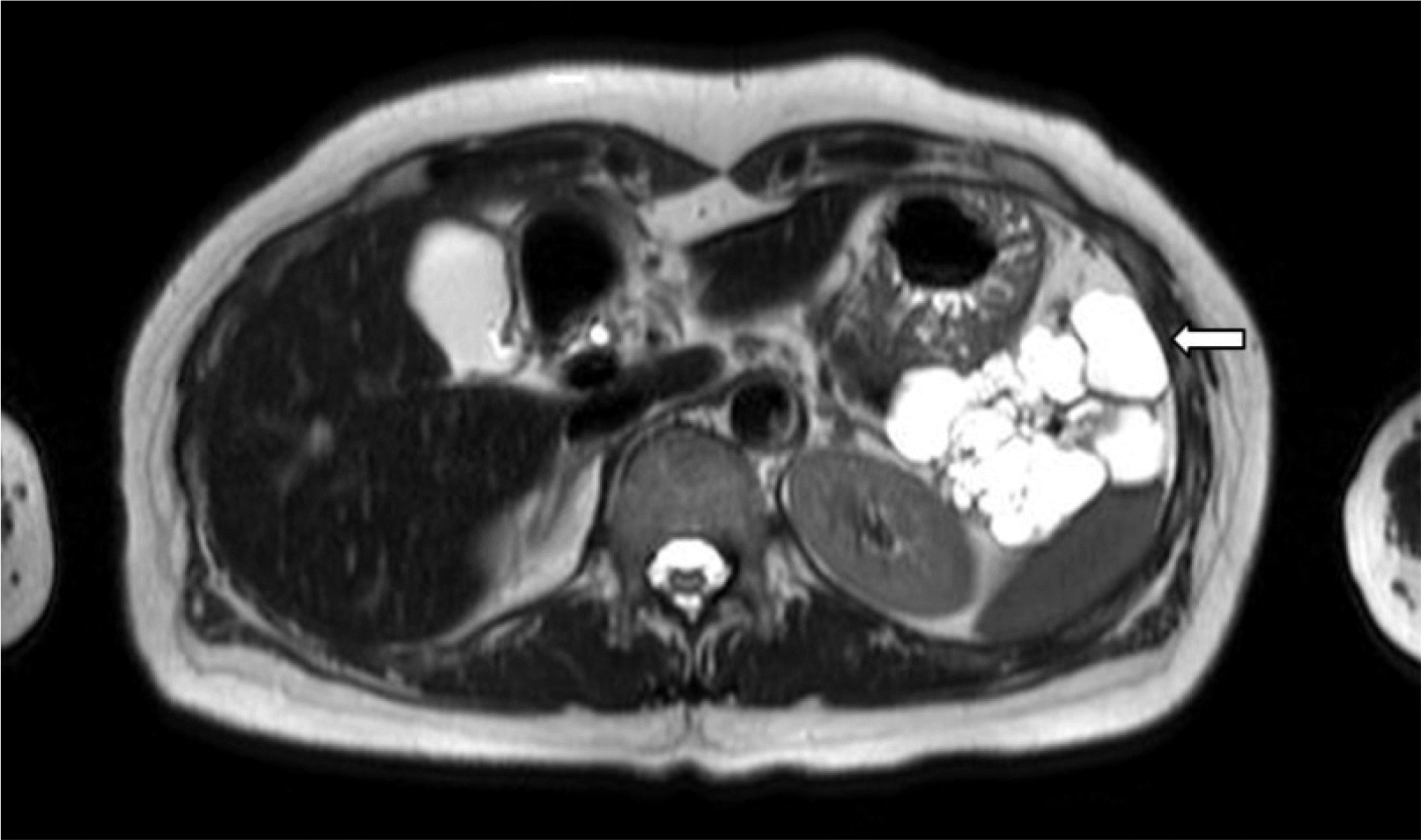
An 8 cm-sized lobulated and multilocular cyst was observed in the pancreatic tail, which was not connected to the main pancreatic duct on MRI performed after she was transferred (Fig. 2).
Serous cystadenoma of the pancreas as large as 8 cm was suspected, and as the patient was anxious, surgery was requested for postoperative confirmation.
The preoperative blood test results were in the normal range as follows: white blood cell count 5,770/mm3 (reference value 4,000-10,000/mm3), hemoglobin 13.9 g/dL (reference value 12.0-16.0 g/dL), total bilirubin 1.1 mg/dL (reference value 0.2-1.2 mg/dL), AST 21 U/L (reference value 13-33 U/L), ALT 16 U/L (reference value 6-27 U/L), ALP 200 U/L (reference value 115-359 U/L), urea nitrogen 19 mg/dL (reference value 8-22 mg/dL), creatinine 0.52 mg/dL (reference value 0.6-0.9 mg/dL), sodium 145 mmol/L (reference value 138-145 mmol/L), potassium 4.2 mmol/L (reference value 3.6-4.8 mmol/L), and chloride 105 mmol/L (reference value 101-108 mmol/L).
Laparoscopic distal pancreatectomy was performed, and a 7.0×4.0×3.0 cm-sized multilocular cystic tumor was observed during a visual examination. The tumor was well distinguished from the normal pancreatic tissue, and the cyst wall was thin and translucent (Fig. 3).
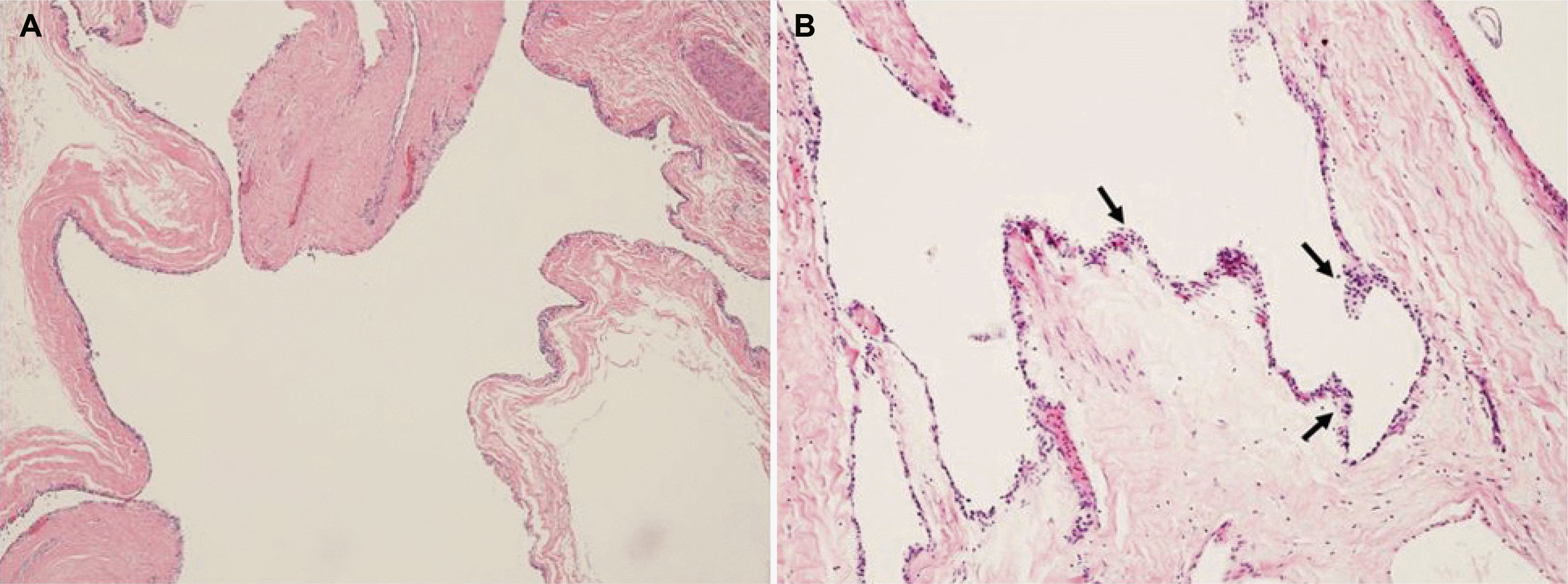
Microscopically, the tumor was composed of variable sized multilocular cysts with incomplete septa. The cysts were interconnected and focally lined by epithelium with acinar differentiation (Fig. 4). The patient was diagnosed with acinar cell cystadenoma based on a histologic findings. The patient is currently being followed up regularly, and there have been no complications or recurrence since surgery.
Go to : 
Acinar cell cystadenoma is a benign cystic lesion of the pancreas that shows acinar cell differentiation defined as the generation of zymogen granules, including the pancreatic exocrine enzyme.3 The condition is also known as an acinar cystic transformation of the pancreas and is very rare.
Klöppel4 described an acinar cystic transformation in 2000, and Albores-Saavedra5 proposed acinar cell cystadenoma to describe a 9 cm-sized multilobular pancreatic cyst found during the autopsy of a 58-year-old woman in 2002. The etiology of acinar cell cystadenoma is unclear; it is more prevalent in women and occurs across different age groups.2,6 It can occur in any part of the pancreas but tends to be found more commonly in the head. It is mostly localized but may exist as diffuse lesions in approximately 10% of patients.2,7 Acinar cell cystadenoma is diagnosed based on a combination of the clinical features, radiological features, and histopathological findings. Clinically, abdominal pain is the most common symptom, but it is often discovered incidentally during an imaging test.2,8
The imaging features are not specific, but Delavaud et al.9 suggested that they are more closely related to acinar cell cystadenoma than to branch duct intraductal papillary mucinous neoplasia based on the following imaging findings: 1) five or more cysts, 2) clustered small, peripheral cysts, 3) presence of cyst calcification, and 4) absence of communication with the main pancreatic duct. The modality showed 100% sensitivity and 60% specificity when at least one criterion is satisfied, 100% sensitivity and 85% specificity when at least two criteria are satisfied, 85% sensitivity and 100% specificity when at least three criteria are satisfied, and 60% sensitivity and 100% specificity when all four criteria are satisfied. This case satisfied three of the imaging findings: five or more cysts, presence of cyst calcification, and absence of communication with the main pancreatic duct.
Although EUS-guided fine needle aspiration (FNA) is used to diagnose approximately 75% of pancreatic cystic tumor patients, it may show false-negative results because of small specimen size, sampling error, and lack of preservation of the tissue architecture. EUS-guided FNA may be helpful in a differential diagnosis before surgery, but the diagnosis is mostly confirmed based on the histopathological testing of the surgical specimen.3
Histopathologically, the cyst wall consists of cells with acinar cell differentiation that lack nuclear mitotic figures, distinct cellular atypia, necrosis, and infiltrative growth.3 The presence of intracellular eosinophilic zymogen granules is an excellent pathological indicator that the lesion may be an acinar cell cystadenoma.10
Acinar cell cystadenoma and serous cystadenoma are difficult to distinguish, especially those with a multilocular and microcystic pattern, as in the present case. Acinar cell cystadenoma is characterized by interconnected and dilated acinar epithelium. The cysts are lined by 1-2 layer flatten or cuboidal epithelium with acinar differentiation.
In immunohistochemical staining, cells in the cyst wall differ from the normal cells. They are positive for acinar cell differentiation markers, such as trypsin and chymotrypsin, and also for CK7, which is negative in normal acinar cells.11
Caution should be taken when distinguishing acinar cell cystadenoma from acinar cell cystadenocarcinoma during a differential diagnosis. Acinar cell cystadenoma can be distinguished from acinar cell cystadenocarcinoma because it shows very low positivity in Ki-67 staining, no dysplasia, and no infiltration into the surrounding tissues.2
A surgical resection is recommended to exclude other cystic neoplasms associated with malignant tumors, prevent local expansion or malignant transformation of the cyst, and relieve the symptoms.8 The prognosis is good, and malignant transformations or recurrences have not been reported.3
The PubMed database was searched from 2000 until the present using a keyword search for “acinar cell cystadenoma” and “acinar cystadenoma” and 69 patients with acinar cell cystadenoma were identified (Table 1).1,3,5-8,10,12-23
Clinical Features of the Reported Cases of Pancreatic Acinar Cell Cystadenoma
This was the case of an 8 cm-sized cystic tumor in the pancreatic tail that was detected accidentally during an abdominal imaging test in a 51-year-old woman without any symptoms. The diagnosis of acinar cell cystadenoma was made after surgery, while serous cystadenoma of the pancreas was suspected from the image. Although only a few cases are being reported as acinar cell cystadenoma, which is a very rare disease, it should be considered in a differential diagnosis of cystic tumors of the pancreas. Here, the authors report one case of acinar cell cystadenoma that was misrecognized as a serous cystadenoma of the pancreas.
Go to : 
REFERENCES
1. Gumus M, Ugras S, Algin O, Gundogdu H. 2011; Acinar cell cystadenoma (acinar cystic transformation) of the pancreas: the radiologic-pathologic features. Korean J Radiol. 12:129–134. DOI: 10.3348/kjr.2011.12.1.129. PMID: 21228949. PMCID: PMC3017877.

2. Kim BH, Park SY, Kim B, Kang GH. 2007; Acinar cell cystadenoma of the pancreas: report of a case with metaplastic ossification. J Pathol Transl Med. 41:203–206.
3. Wang G, Ji L, Qu FZ, et al. 2016; Acinar cell cystadenoma of the pancreas: a retrospective analysis of ten-year experience from a single academic institution. Pancreatology. 16:625–631. DOI: 10.1016/j.pan.2016.03.020. PMID: 27086062.

4. Klöppel G. 2000; Pseudocysts and other non-neoplastic cysts of the pancreas. Semin Diagn Pathol. 17:7–15. PMID: 10721803.
5. Albores-Saavedra J. 2002; Acinar cystadenoma of the pancreas: a previously undescribed tumor. Ann Diagn Pathol. 6:113–115. DOI: 10.1053/adpa.2002.32379. PMID: 12004359.

6. Singhi AD, Norwood S, Liu TC, et al. 2013; Acinar cell cystadenoma of the pancreas: a benign neoplasm or non-neoplastic ballooning of acinar and ductal epithelium? Am J Surg Pathol. 37:1329–1335. DOI: 10.1097/PAS.0b013e3182a1ad72. PMID: 24076773.
7. Tanaka H, Hatsuno T, Kinoshita M, et al. 2016; A resected case of symptomatic acinar cell cystadenoma of the pancreas displacing the main pancreatic duct. Surg Case Rep. 2:39. DOI: 10.1186/s40792-016-0166-1. PMID: 27108123. PMCID: PMC4842199.

8. Wolf AM, Shirley LA, Winter JM, et al. 2013; Acinar cell cystadenoma of the pancreas: report of three cases and literature review. J Gastrointest Surg. 17:1322–1326. DOI: 10.1007/s11605-013-2199-0. PMID: 23605178.

9. Delavaud C, Cros J, et al. d'Assignies G. 2014; CT and MR imaging of multilocular acinar cell cystadenoma: comparison with branch duct intraductal papillary mucinous neoplasia (IPMNs). Eur Radiol. 24:2128–2136. DOI: 10.1007/s00330-014-3248-0. PMID: 24895037.

10. Sopha SC, Terhune JH, Hoover L, Uradomo L, Boutros CN. 2018; Acinar cell cystadenoma of the pancreas: a multidisciplinary and contemporary approach. J Gastrointest Surg. 22:1797–1798. DOI: 10.1007/s11605-018-3698-9. PMID: 29380117.

11. Volkan Adsay N. 2007; Cystic lesions of the pancreas. Mod Pathol. 20 Suppl 1:S71–S93. DOI: 10.1038/modpathol.3800706. PMID: 17486054.

12. Chen AL, Misdraji J, Brugge WR, Ferrone CR, Pitman MB. 2017; Acinar cell cystadenoma: a challenging cytology diagnosis, facilitated by moray® micro-forceps biopsy. Diagn Cytopathol. 45:557–560. DOI: 10.1002/dc.23693. PMID: 28236434.

13. Sigel CS, Klimstra DS. 2013; Cytomorphologic and immunophenotypical features of acinar cell neoplasms of the pancreas. Cancer Cytopathol. 121:459–470. DOI: 10.1002/cncy.21279. PMID: 23408736.

14. Orr J, Lockwood R, Roberts J, Shi C, Yachimski P. 2018; EUS and confocal endomicroscopic diagnosis of pancreatic acinar cell cystadenoma. Gastrointest Endosc. 88:769–770. DOI: 10.1016/j.gie.2018.06.003. PMID: 29906415.

15. Bergmann F, Aulmann S, Welsch T, et al. 2014; Molecular analysis of pancreatic acinar cell cystadenomas: evidence of a non-neoplastic nature. Oncol Lett. 8:852–858. DOI: 10.3892/ol.2014.2163. PMID: 25009661. PMCID: PMC4081433.

16. Zhang X, Zhu H, Yang X, Adsay VN, Jain D. 2017; Post-obstructive cyst formation in pancreas and cystic acinar transformation: Are they same? Pathol Res Pract. 213:997–1001. DOI: 10.1016/j.prp.2017.03.013. PMID: 28599853.

17. Alkhateeb MA, Boqari D, Mansi NK. 2020; Pancreatic acinar cystadenoma in a background of diffuse multifocal pancreatic cystic lesions: a case report. Int J Surg Case Rep. 73:223–227. DOI: 10.1016/j.ijscr.2020.07.026. PMID: 32712551. PMCID: PMC7390793.

18. Fahlbusch T, Tannapfel A, Uhl W, Braumann C. 2018; Acinar cell cystadenoma - a rarity in advanced von Hippel-Lindau disease: a case report. Visc Med. 34:73–75. DOI: 10.1159/000480372. PMID: 29594173. PMCID: PMC5869601.

19. Cosgrove N, DiPalma J, Katz D, Kowalski T. 2016; A rare case of acinar cell cystadenoma in a 14-year-old adolescent: a case report. Case Rep Pancreat Cancer. 2:3–5. DOI: 10.1089/crpc.2015.29009.nco. PMID: 30631807. PMCID: PMC6319691.

20. Khor TS, Badizadegan K, Ferrone C, et al. 2012; Acinar cystadenoma of the pancreas: a clinicopathologic study of 10 cases including multilocular lesions with mural nodules. Am J Surg Pathol. 36:1579–1591. DOI: 10.1097/PAS.0b013e318265fa4b. PMID: 23060352.
21. Rift CV, Hasselby JP, Hansen CP, Federspiel B. 2020; Acinar cystic transformation of the pancreas: report of a case and a review of the literature. Pathol Res Pract. 216:152928. DOI: 10.1016/j.prp.2020.152928. PMID: 32204924.

22. Scott BB, Price TP, Callahan ZM, Poling JS, Lavu H. 2016; Intraductal papillary mucinous neoplasm of the pancreas arising in the setting of an intermixed acinar cell cystadenoma of the pancreas: report of a rare case. Case Rep Pancreat Cancer. 2:75–78. DOI: 10.1089/crpc.2016.0018. PMID: 30631822. PMCID: PMC6319694.

23. Wen X, Bandovic J. 2020; Fifteen-year follow-up of a patient with acinar cystic transformation of the pancreas and literature review. Case Rep Pathol. 2020:8847550. DOI: 10.1155/2020/8847550. PMID: 33425418. PMCID: PMC7781703.

Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download