The tumor biology of malignant melanoma is highly unpredictable. Although melanoma may go into spontaneous remission, it can also cause systemic metastases after it is thought to be cured [
2]. Melanoma is considerably more likely to regress than all other cancers. Approximately 4% to 6% of metastatic melanomas present with an unknown primary lesion [
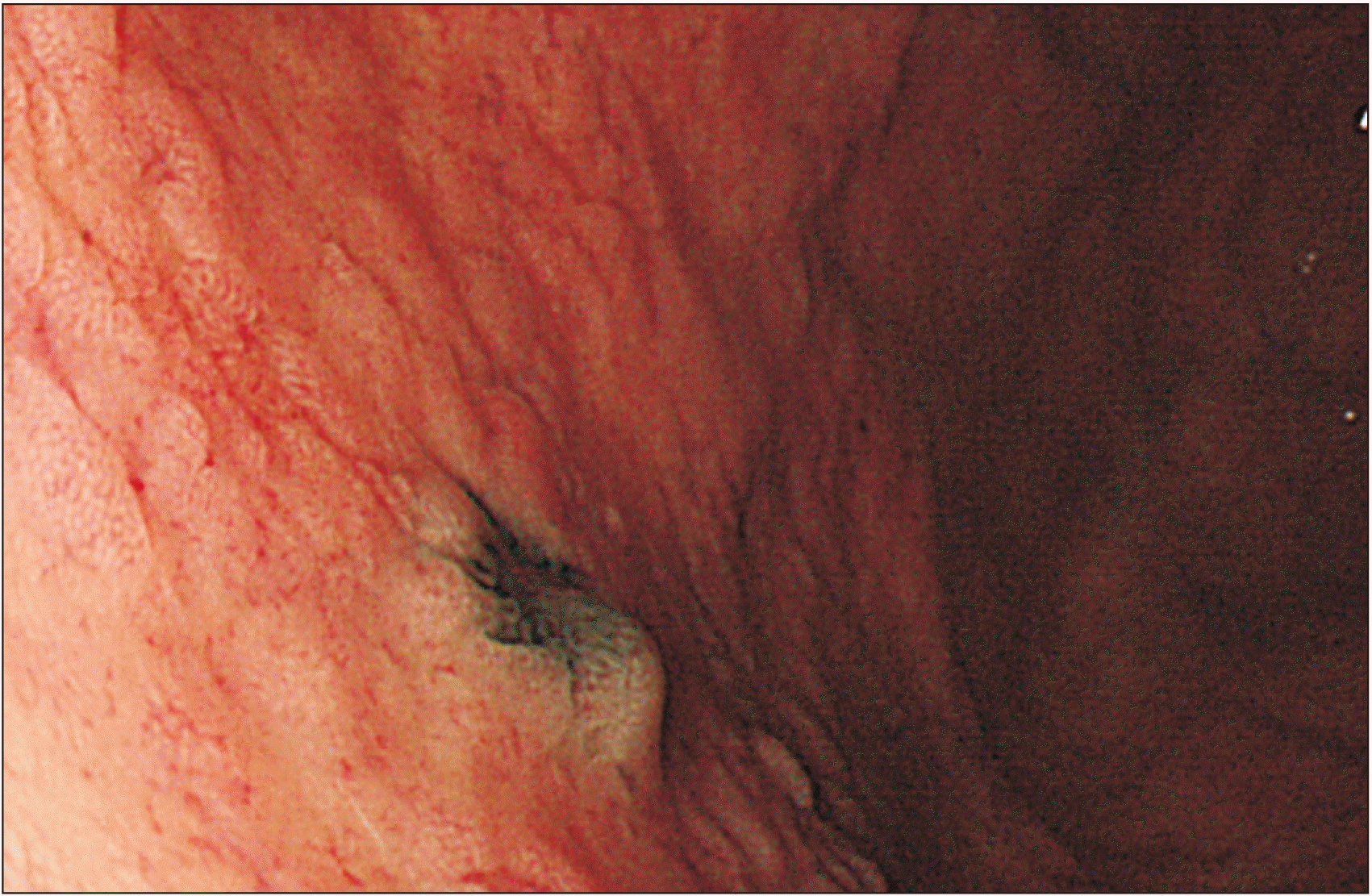
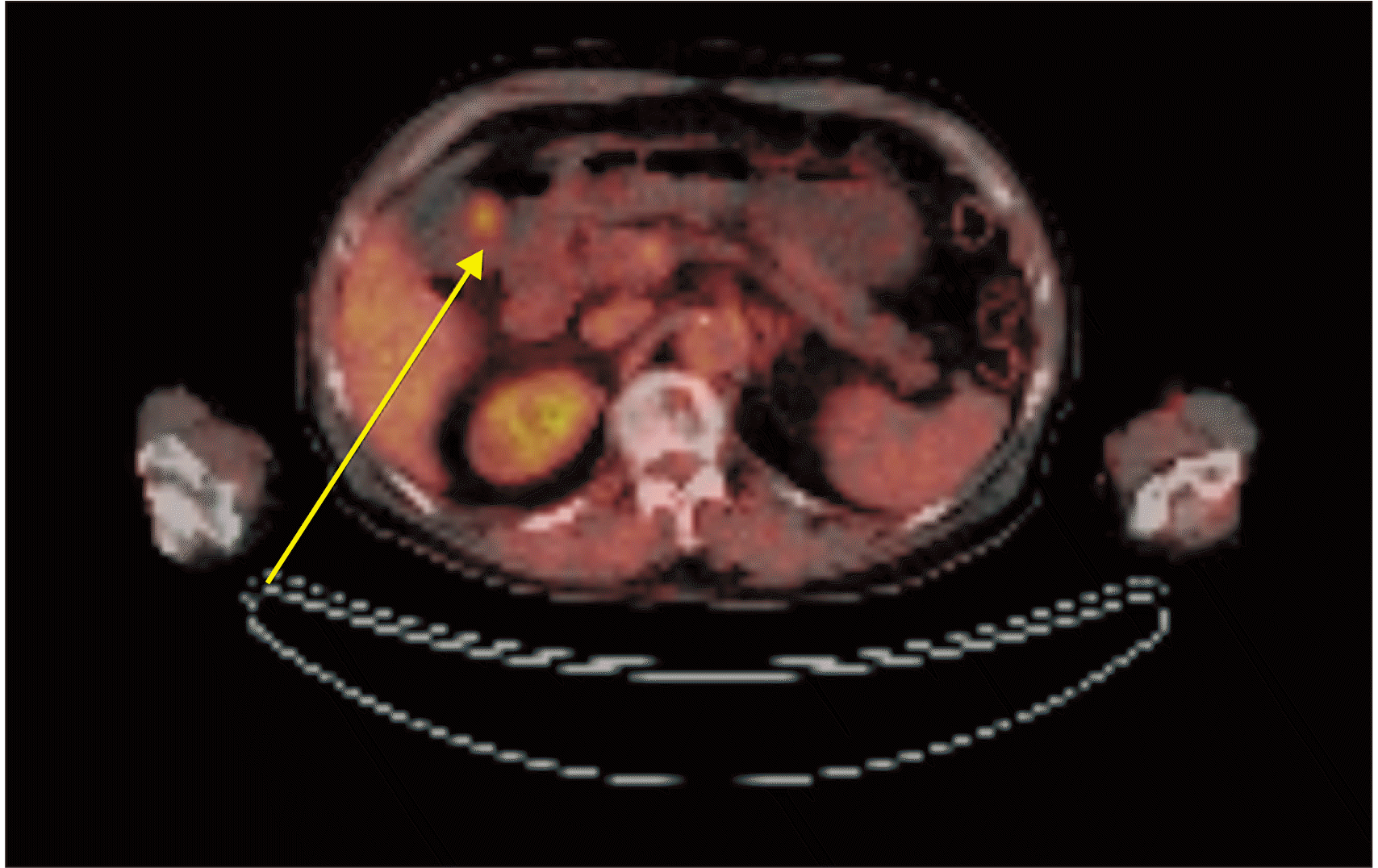
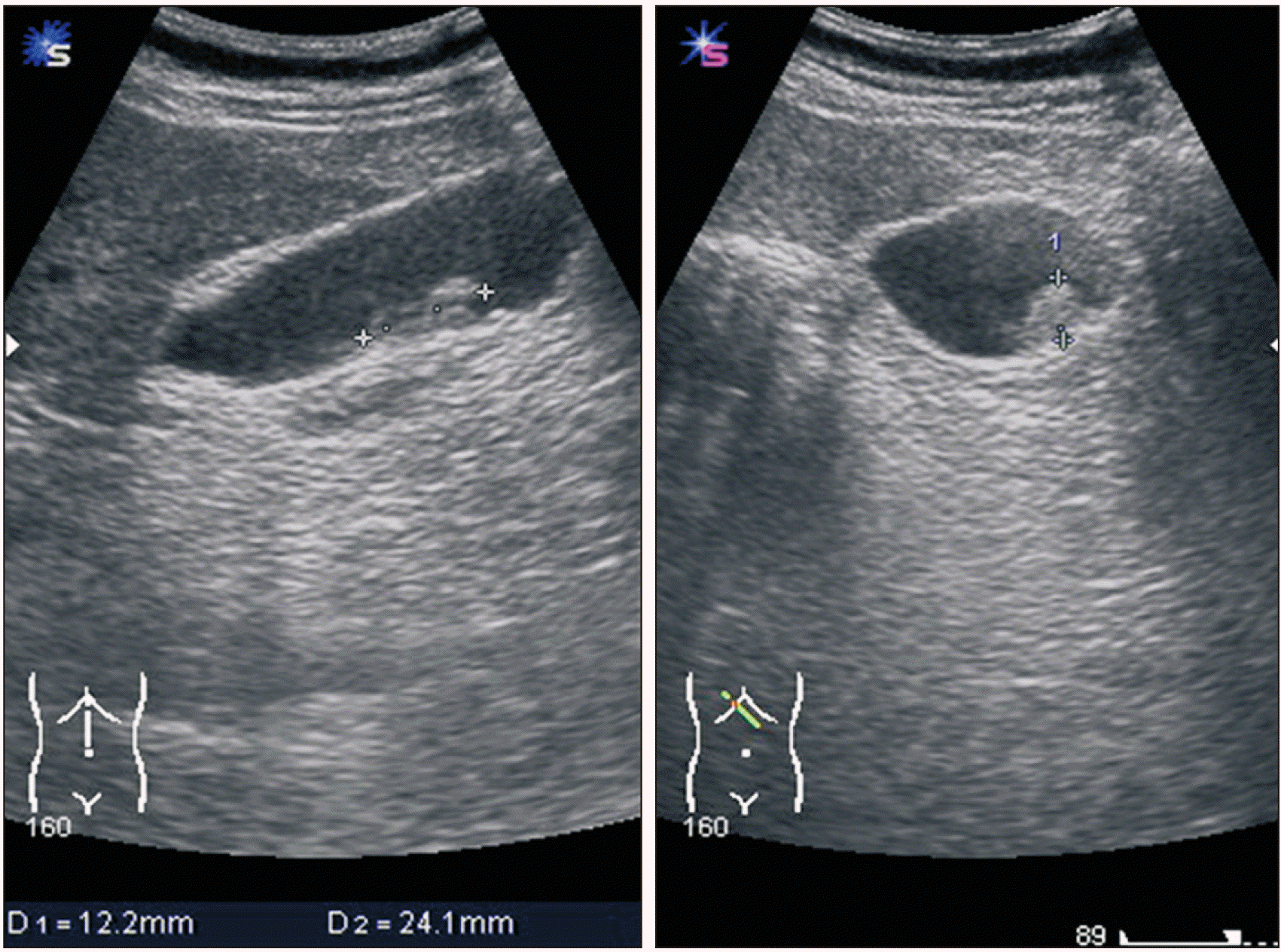
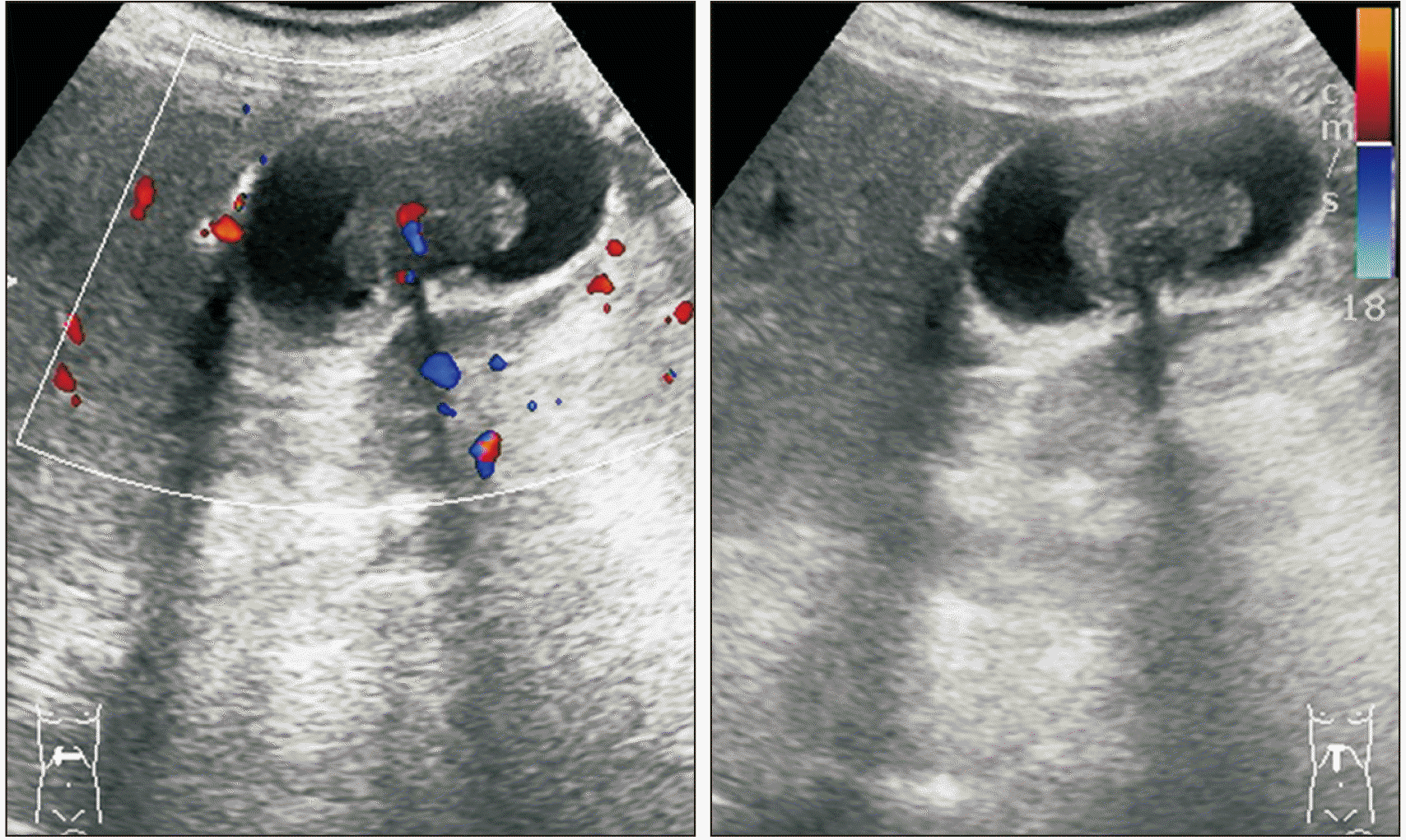
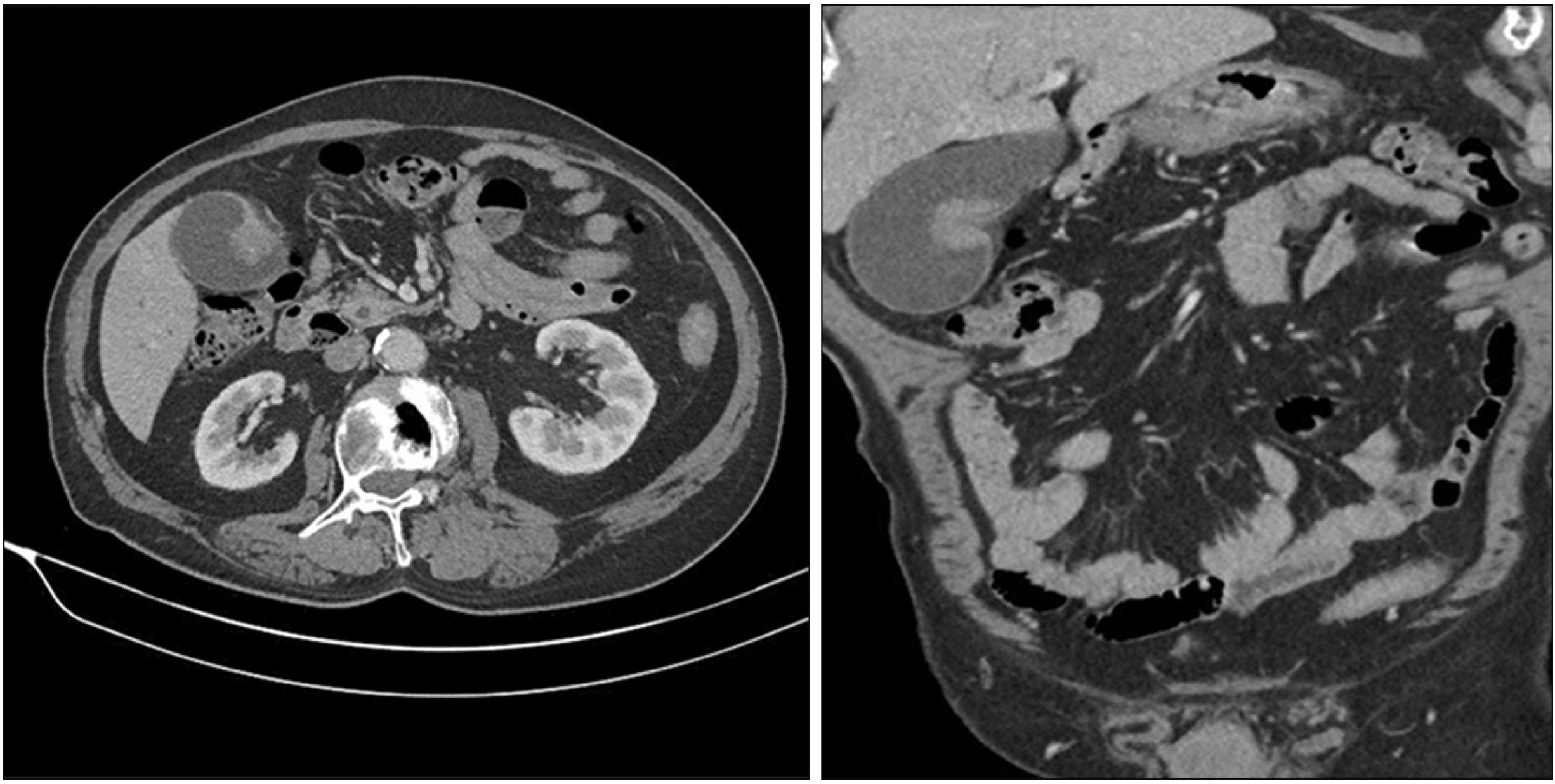
1]. In our case, the pathologist indicated that the lesion in the stomach was a metastatic disease and recommended us to look for the primary lesion. Extensive examinations were conducted, including ophthalmology, otorhinolaryngology, and dermatology exams. However, the causative lesion was not found. Dermatological examination revealed hypopigmentation in the anterior chest and left leg. Punch biopsy was performed to determine the possibility of complete regression of melanoma. However, chronic dermatitis was confirmed. The specimen suggested that the primary lesion was either an unknown melanoma or originated in the stomach.
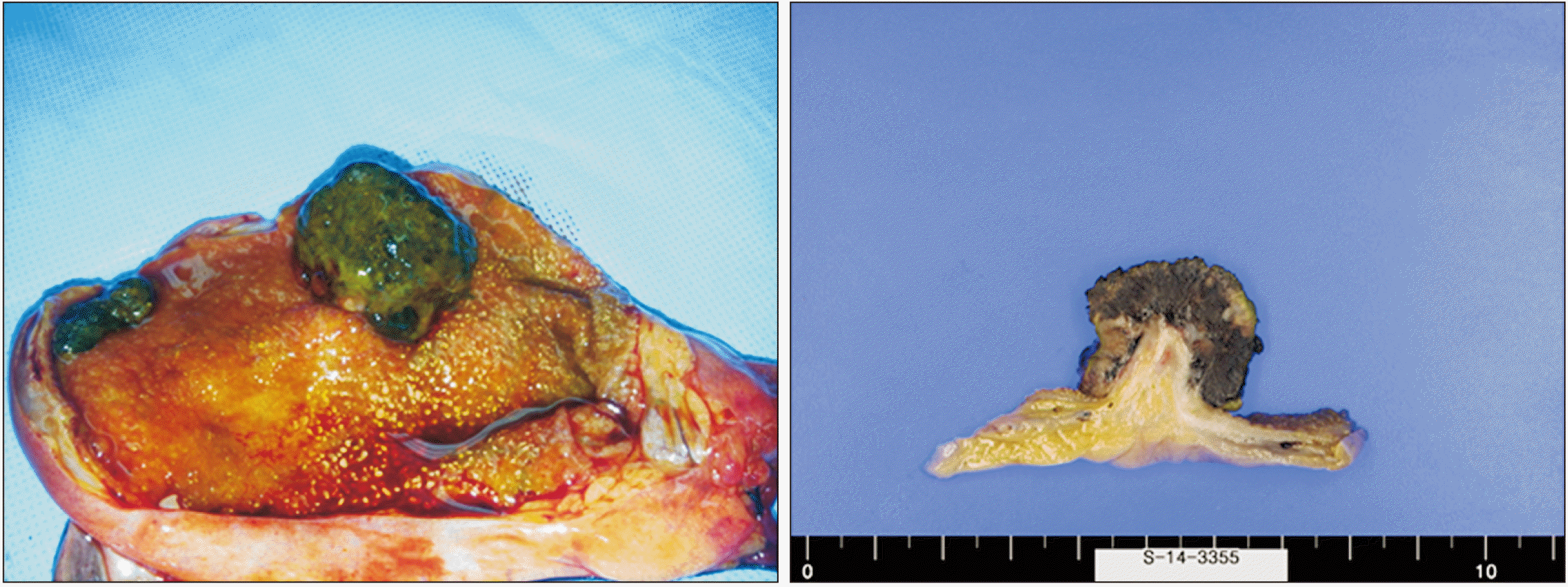
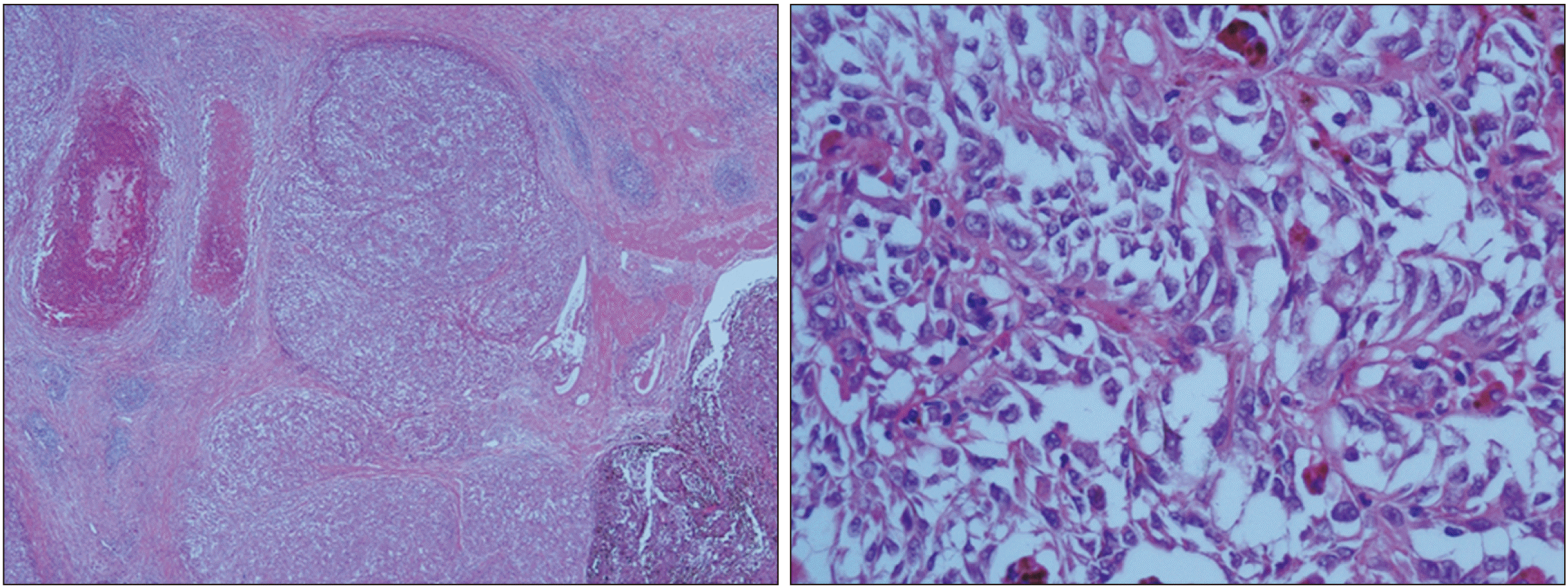
The criteria for distinguishing whether a gallbladder tumor is a primary or metastatic lesion are difficult. Heath and Womack have proposed criteria for distinguishing primary melanoma from metastatic melanoma [
3]. They proposed that primary tumors must be 1) solitary arising from the mucosal surface; 2) papillary or polypoid; 3) displaying junctional activity (the presence of pigmented dendritic cells at the junction of the epithelium and lamina propria); and 4) having other sites excluded as the primary site of origin. Among these criteria, junctional activity is considered the most indicative of a primary lesion. Our case presented with a solitary polypoid lesion. However, there was insufficient evidence to diagnose primary gallbladder melanoma because junctional activity could not be assessed. Cutaneous melanoma is known to metastasize to any organ. Metastatic disease from this malignancy to the gastrointestinal tract occurs at a frequency of 2% to 4%. Of these cases, the gallbladder is found to be a metastatic site at a frequency of 15% [
4]. When referring to the previous literature, it is reasonable to consider that a gallbladder lesion is the metastatic lesion. The prognosis of melanoma of the gallbladder is very poor. Mean survival rates for patients with primary and metastatic lesions are 20.1 months and 8.4 months, respectively [
5]. Primary melanoma of the gallbladder has a better prognosis than metastatic melanoma of the gallbladder because it is usually accompanied by symptoms such as acute cholecystitis confined to the gallbladder [
6]. Treatment options for a metastatic disease depend on the extent of the disease and the clinical manifestation in the patient. Six case reviews of metastatic melanoma of the gallbladder have been reported (
Table 1). Katz et al. [
6] reported 13 cases of metastatic melanoma of the gallbladder in 2007. Patients who presented with disease confined to the gallbladder had a median survival of 39 months compared to a median survival of 10 months for patients presenting with multiple metastatic sites. Among patients who underwent cholecystectomy, there was a 10-month increase in survival compared to those who did not. The authors emphasized that factors associated with an improved survival rate were symptom manifestation, isolated metastases, and surgical approach. They suggested that, considering the aggressive nature and the biology of advanced melanoma, more extensive surgical resections such as lymphadenectomy did not seem to be warranted. In this study, three laparoscopic cholecystectomies were performed and two cases of port metastases occurred, although a retrieval bag was used to avoid dissemination of cancer cells. Tuveri and Tuveri [
7] have reported a case of isolated metastatic melanoma to the gallbladder treated by laparoscopic cholecystectomy and lymphadenectomy of the hepatoduodenal ligament. They suggested that laparoscopic cholecystectomy could be used as an adequate treatment once the presence of a widespread disease was ruled out. The surgeon should use a retrieval bag for the removal of the gallbladder to avoid disruption of the gallbladder, which could cause port site or peritoneal metastases. Most gallbladder metastases of melanoma are intraluminal. Lymphadenectomy is not appropriate for stage IV melanoma [
8]. However, this study notes that if the diagnosis of gallbladder lesions is unclear (primary or metastasis), the patient is young, or the extent of the tumor is large, lymphadenectomy may be considered. They recommended that lymphadenectomy should be performed only by experienced laparoscopic surgeons to reduce the rate of complications [
7]. Christou et al. [
9] have reported a case of metastatic malignant melanoma of the gallbladder accompanied by lymphadenopathy of the celiac axis and liver hilum. They performed open cholecystectomy and wedge liver resection and removed only suspected lymph nodes that were palpable intraoperatively. Marone et al. [
10] and Giannini et al. [
11] have also removed only suspected lymph nodes based on preoperative imaging or surgical findings. However, since the final biopsy results were different from their expectations and the exact lymph node metastasis could not be determined, adequate lymph node dissection is recommended. The patient in the present case report was presumed to have gallbladder cancer. Thus, surgery was performed. However, the lesion was diagnosed as melanoma with lymph node metastasis. It was considered a metastatic gallbladder melanoma because of his previous melanoma diagnosis history. In the case of stage IV melanoma, it is not appropriate to consider lymph node dissection for a palliative surgery. However, in our case, a long-term survival rate was confirmed. A reliable indicator for the differential diagnosis between primary and metastatic melanoma is junctional activity in the pathology report. However, this is difficult to accurately diagnose with frozen biopsy specimens during surgery. Considering the uncertainty of the diagnosis and the long-term survival of our patient, we think that performing lymphadenectomy after considering the general condition of the patient and/or tumor extent is a suitable option. Although lymphadenopathy was not identified in the preoperative imaging study, metastasis was confirmed for the lymph nodes obtained during the surgery. Therefore, we expect it would be better to have enough lymph node dissection. Recurrence of laparoscopic port sites has not been reported outside the review paper of Katz et al. [
6]. Thus, if there are no other organ metastases and if the lesion is confined to the gallbladder, laparoscopic cholecystectomy may be considered. Endo-bags should be considered to prevent cancer cells from contaminating the abdominal cavity. However, if aggressive surgical treatment is planned after considering the patient’s age and/or general condition rather than palliative surgery for symptom control or prophylactic cholecystectomy, we think it would be better to have an open surgery for adequate lymph node dissection.
Table 1
Summary of clinical features of previous reports of metastatic melanoma of the gallbladder
|
Author |
Year |
Age (yr) |
Sex |
Site of primary |
Other metastatic organ |
Treatment |
Open or laparoscopic surgery |
Lymphadenectomy |
Lymph node metastasis |
Overall survival after surgery |
|
Guida et al. [5] |
2002 |
32 |
F |
Rt. shoulder |
(-) |
Cholecystectomy |
Open |
Locoregional lymphadenectomy |
Hepatoduodenal ligament LN (+) |
4 mona)
|
|
Katz et al. [6] |
2007 |
Not described |
F: 6 M: 7 |
Trunk 7, Head & neck 3, Extremity 1, Vulva 1, Unknown origin 1 |
Multiple metastasis: 8 (liver, lung, bone, brain adrenal, thyroid) |
Cholecystectomy: 9 Non-operative treatment: 4 |
Open: 6 Lap.: 3 |
Not described |
Not described |
Cholecystectomy– median 16 mon Non-operative- median 6 mon |
|
Marone et al. [10] |
2007 |
54 |
M |
Trunk |
Diaphragmatic peritoneum |
Cholecystectomy, CTX, ITX |
Lap. |
Suspected LN |
LN (+) |
11 mon |
|
Tuveri and Tuveri [7] |
2007 |
37 |
F |
Rt. leg |
(-) |
Cholecystectomy |
Lap. |
Locoregional lymphadenectomy |
LN (-) |
More than 5 years |
|
Christou et al. [9] |
2014 |
58 |
M |
Back |
Liver hilum |
Cholecystectomy Wide liver wedge resection |
Open |
Suspected LN |
LN (-) |
More than 6 mona)
|
|
Giannini et al. [11] |
2016 |
50 |
M |
Back |
Spleen |
Cholecystectomy Splenectomy |
Lap. |
Suspected LN |
LN (-) |
More than 6 mona)
|
|
|
40 |
F |
Rt. hemithorax |
(-) |
Cholecystectomy |
Lap. |
Suspected LN |
LN (-) |
More than 6 mona)
|

In conclusion, in case of melanoma found in the gallbladder, the original melanoma lesion has a possibility of regression. Since biopsy could determine whether it is a primary or metastatic lesion, making treatment planning very difficult. Treatment guidelines for melanoma found in the gallbladder are unclear due to its low occurrence. We report a rare case of metastatic malignant melanoma of the gallbladder treated with an open radical cholecystectomy, including lymphadenectomy of regional lymph nodes and liver wedge resection. Even for cases of metastatic melanoma discovered by chance, considering the long-term survival of our case following a surgical resection, extended cholecystectomy including regional lymph node dissection may be a good therapeutic approach for gallbladder melanoma.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download