This article has been
cited by other articles in ScienceCentral.
Abstract
Bladder cancer is the 9th most frequent cancer worldwide. Its incidence is increasing. The pancreas is an infrequent site of metastasis in relation to any type of malignancy. In this study, we report our experience with a patient who has undergone a pancreaticoduodenectomy for metastatic bladder cancer. A 61-year-old male was admitted with jaundice and pancreas head mass. He underwent robot assisted-cystectomy and ileal conduit for bladder cancer 7 months ago. Initial diagnosis under the imaging study was a resectable pancreas head cancer. However, we did not rule-out a metastatic bladder cancer. He underwent a classic pancreaticoduodenectomy. Based on histologic findings and immunohistochemistry results, a pancreas tumor with 4.9-cm sized metastatic urothelial carcinoma was diagnosed. He experienced no complication. He was discharged 11 days after the surgery. Four cycles of gemcitabine and cisplatin were administered. He remained recurrence-free of tumors for 16 months. Although the benefit of pancreatectomy for patient survival has been reported for metastases from renal cell carcinoma, it is unknown for bladder cancer because of no report. We believe that curative resection for metastasis to pancreas of urothelial carcinoma might be helpful for its management.
Go to :

Keywords: Uurinary bladder neoplasm, Neoplasm metastasis, Pancreaticoduodenectomy, Pancreatic neoplasm
INTRODUCTION
Bladder cancer is the 9th most frequent cancer worldwide. Its incidence is increasing [
1]. Urothelial carcinoma accounts for 90% of cases in Western countries. Squamous cell carcinoma is the most common bladder cancer in eastern Africa and the Middle East. Aging, smoking, schistosomiasis infection, and occupational exposures such as exposure to aromatic amines are well-known causes [
2]. In Korea, the number of bladder cancer cases continuously increased from 2,180 cases in 1999 to 3,549 cases in 2011, a total of 37,950 cases during the period [
3]. Radical cystectomy with pelvic lymph node dissection and urinary division has been a standard curative treatment for muscle-invasive bladder cancer. With advances in various imaging modalities, earlier detection of recurrent and metastatic disease is possible. Although bladder cancer is one of the most common malignant diseases, its metastatic pattern and benefit of additional operation are limited. Shinagare et al. [
4] have reported that 47 patients among 150 cases have isolated organ involvement, including the lymph node, bone, lung, peritoneum, liver, and brain (in descending order of frequency), with pancreas metastasis having only one case. Operative treatment is very rare for pancreas metastases from a bladder cancer. There is no report of its benefit for such disease entity. In this study, we report our experience of pancreatoduodenectomy for pancreas metastases from a bladder cancer.
Go to :

CASE
A 61-year-old male was admitted with jaundice and pancreas head mass. He underwent robot assisted-cystectomy and ileal conduit for bladder cancer 7 months ago. Histologic result of bladder cancer was high grade by World Health Organization (WHO)/International Society of Urologic Pathologist 2004 and Grade III by WHO 1973. According to American Joint Committee on Cancer 8th, it was T3N0 without marginal involvement. However, it showed lymph-vascular invasion and perineural invasion. His total bilirubin and carbohydrate antigen 19-9 levels were 25.13 mg/dL (normal range, 0.1–1.2 mg/dL) and 248 U/mL (normal range, 0–39 U/mL) upon admission. He received endoscopic retrograde cholangiopancreatography and biliary drainage for diagnosis and treatment. His total bilirubin level was 6.1 mg/dL at operation. Contrast enhanced computed tomography images demonstrated a 3.6-cm sized well-defined mass with dilation of upstream bile duct and pancreatic duct. On magnetic resonance imaging, a well-defined hypodense mass on the pancreas head with a double duct sign was found (
Fig. 1). Initial diagnosis under the imaging study was resectable pancreas head cancer. However, we did not rule-out the metastatic bladder cancer. He underwent a classic pancreaticoduodenectomy. The pathologic diagnosis for this patient was 4.9-cm sized metastatic urothelial carcinoma with lymph node metastasis (4/24) (
Fig. 2). On microscopic finding, the mass consisted of poorly differentiated round to oval shaped cells with high grade dysplasia. Tumor cells infiltrated into the pancreas parenchyma, duodenum wall, common bile duct wall, and ampulla of Vater. Most neoplastic cell growth solid pattern and some glandular structures were identified. The differential diagnosis included poorly differentiated pancreas ductal adenocarcinoma and neuroendocrine carcinoma. Because the patient had a high grade urothelial carcinoma history, metastatic urothelial carcinoma was also considered. We performed immunohistochemistry studies to determine tumor cell differentiation. Tumor cells were negative for neuroendocrine markers (synaptophysin, chromogranin, CD56) but positive for epithelial markers (CK7, CK19) (
Fig. 3). The original urothelial carcinoma tissue was reviewed. Results were compared to histologic findings and immunohistochemistry results (
Fig. 4). Both tumors had the same histologic findings and immunohistochemistry results. Pancreas tumor cells were diffuse positive for GATA binding protein 3 (GATA-3), a urothelial carcinoma marker in an additional immunohistochemistry study (
Fig. 3). Based on histologic findings and immunohistochemistry results, the pancreas tumor was diagnosed as metastatic urothelial carcinoma. He experienced no complication. He was discharged at 11 days after the surgery. Four cycles of gemcitabine and cisplatin were administered. He remains recurrence-free of tumors currently at 16 months after the surgery.
 | Fig. 1(A, B) Contrast enhanced computed tomography images demonstrating a 3.6-cm sized well-defined mass with dilation of the upstream bile duct and the pancreatic duct. (C–F) On magnetic resonance imaging, the pancreatic mass demonstrated iso signal intensity and low signal intensity on T2WI and T1WI, respectively. The pancreatic mass with a well-defined margin also demonstrated a double duct sign. However, there was no evidence of adjacent vessel invasion. 
|
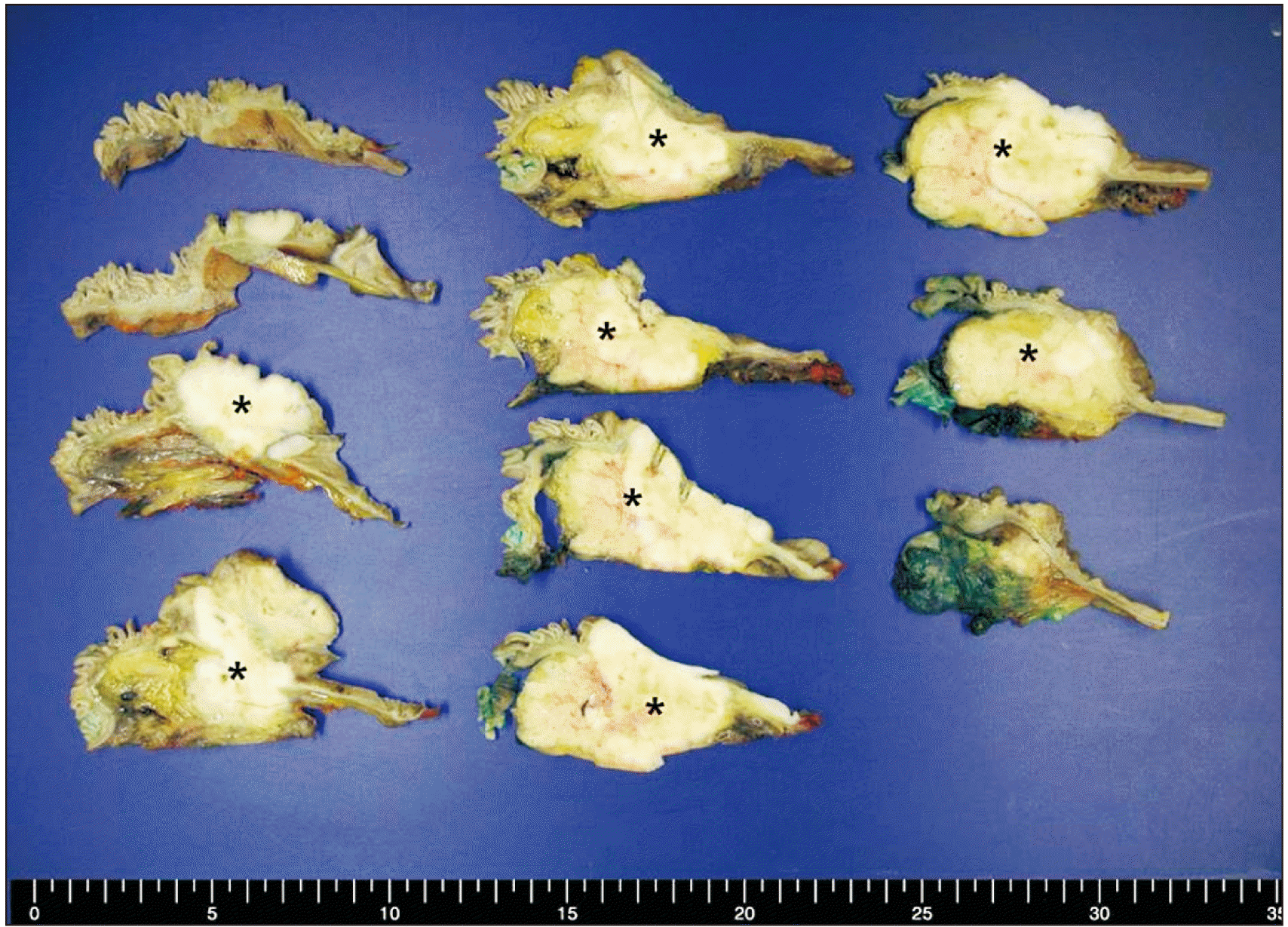 | Fig. 2Gross findings of the cross-sectioned pancreas head from pancreaticoduodenectomy. A relatively well-defined yellow white solid mass was identified (asterisks). The mass involved pancreas head parenchyma, duodenum, and ampulla of Vater. 
|
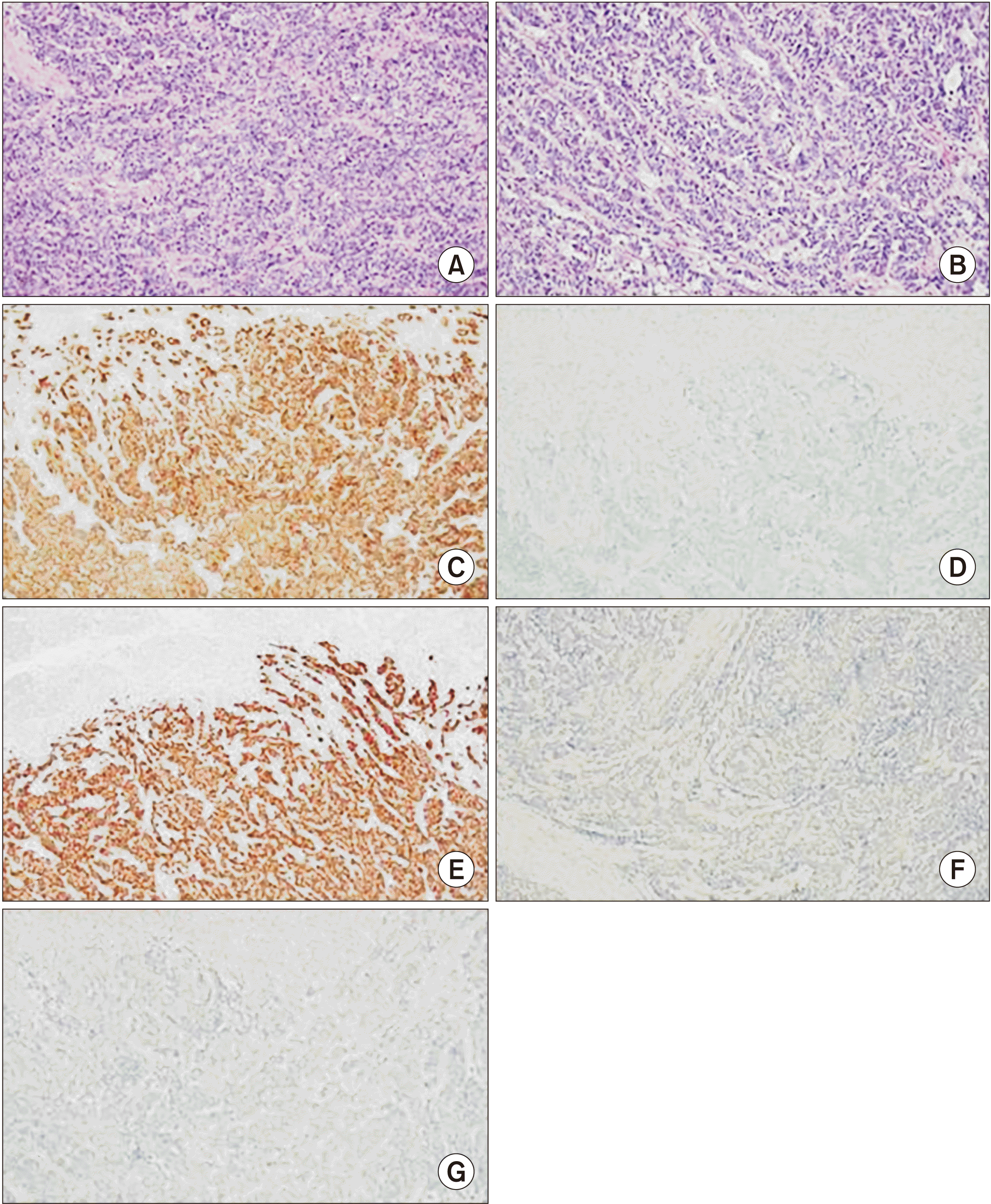 | Fig. 3Microscopic findings of pancreas head tumor. (A, B) Hematoxylin-eosin-stained slide of a representative tumor area showed malignant tumor cells with solid nests (A) or glandular structure (B) similar to urothelial carcinoma. Immunohistochemistry study revealed that tumor cells were positive for CK7 (C) and GATA-3 (E) but negative for CK20 (D), p63 (F), and PAX-8 (G) (A, B: ×200; C–G: ×100). 
|
 | Fig. 4Microscopic findings of infiltrating urothelial carcinoma. (A, B) Urothelial carcinoma cells showed high grade nuclear atypia and solid tumor nests (A) or glandular structures (B). Urothelial carcinoma cells were diffuse positive for CK7 (C) and GATA-3 (F) but negative for p63 (G) and PAX-8 (H) in immunohistochemistry studies. Most of tumor cells were also negative for CK20 (D). However, 10% of tumor cells were positive for CK20 (E) (A, B: ×200; C–H: ×100). 
|
Go to :

DISCUSSION
Pancreas metastases from other primary cancers are rare, accounting for approximately 2% of all pancreatic cancers [
5]. The most frequent primary tumor locations are the kidney, breast, colon, skin, and lung. Bladder cancer has variable metastatic potential. Almost any organ can be involved by metastasis. In bladder cancer, the incidence of pancreas metastases is about 1% [
4]. Generally, pancreatic metastases appear to correlate with a poor prognosis and an early progression of disseminated disease after pancreatic metastasis resection. Outcomes of patients after a surgical resection depend on clinical and biological characteristics of the primary malignancy. Pancreatic renal cell carcinoma metastasis resection has been reported to be associated with a favorable prognosis. However, in contrast with liver and lung metastases, control studies investigating prognosis in the absence of resection have not been reported in the previous research literature. As such, resection remains a controversial therapeutic option. With advances in imaging, earlier detection of recurrent and metastatic disease is possible. However, preoperative differential diagnosis of a primary pancreatic neoplasm and a metastatic tumor can be difficult and somewhat challenging, especially for metachronous cases. Sole metastatic lesions in the pancreas following radical cystectomy are uncommon. Pancreatic metastasis typically takes a long time to occur. As a result, it can be difficult to distinguish a metastatic mass from a neuroendocrine tumor or a pancreatic cancer.
In our case, initial diagnosis under the imaging study was resectable pancreas head cancer. However, we did not rule out a metastatic bladder cancer because of his previous cancer history. Pathologically, the pancreas tumor was diagnosed as a metastatic urothelial carcinoma. We used urothelial carcinoma markers GATA-3 and p63. However, these markers are also expressed in other cancer types [
6,
7]. A high grade urothelial carcinoma can show various morphological and immunohistochemical staining patterns [
8]. We performed multiple immunohistochemistry panels and compared results with those of the original tumor for defined differentiation. Finally, both pancreas and urothelial carcinoma showed similar immunohistochemical staining (CK7+/CK20–/GATA-3+/p63–), supporting the diagnosis.
Our patient was treated with chemotherapy. He remains disease-free at 16 months after the surgery. Although surgical resection for local tumor control depends on clinical and biological characteristics of the primary malignancy, it may be recommended when the lesion is single for patients with a good performance status. Although the benefit of pancreatectomy in terms of patient survival has been reported for metastases from renal cell carcinoma, it is unknown for bladder cancer because of no report. We believe that curative resection for metastasis to the pancreas of a urothelial carcinoma might be helpful for management.
Go to :









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download