This article has been
cited by other articles in ScienceCentral.
Abstract
Pancreatoblastoma (PB) is a rare malignant tumor in adults. It has an overall incidence of 0.004 per 100,000 inhabitants. Its diagnosis with fine-needle aspiration (FNA) is difficult due to multiple differentiation lines present on PB that overlap with other tumors. A 76-year-old male patient presented with jaundice, weight loss of 10 kg in 6 months, and appetite loss. Abdominal computed tomography scan showed a tumor in the pancreas head. Transendoscopic ultrasound with FNA biopsy revealed a malignant epithelial neoplasia compatible with PB with immunohistochemistry CK19 (+), P63(+), synaptophysin (–), and Ki67 50%. The patient underwent surgical resection and adjuvant chemotherapy. Currently, the patient is in follow-up with the latest imaging showing no evidence of disease at 32 months after his initial diagnosis. PB is an uncommon malignant neoplasia with an aggressive behavior. Its diagnostic and therapeutic protocols are unclear. Its preoperative diagnosis may be difficult since its imaging features and serological markers are non-specific. However, FNA may be useful in some situations. Surgical resection is currently the recommended treatment that is associated with the best long-term survival.
Go to :

Keywords: Pancreatoblastoma, Pancreas surgery, Whipple, Pancreatoduodenectomy
INTRODUCTION
Pancreatoblastoma (PB) is a neoplasm of the pancreas that histologically appears similar to fetal pancreatic tissue. In the pediatric population, it accounts for 25% of childhood pancreatic neoplasms. However, adult PB is an infrequent lesion with an incidence of 0.004 per 100,000 inhabitants. It represents less than 1% of all pancreatic neoplasms. To the best of our knowledge, there are less than thirty cases reported in the literature [
1]. PB has a preference for the head of the pancreas in almost 50% of cases. Its size ranges from 1.5 to 20 cm. Mass lesions can cause compressive symptoms, mainly abdominal pain [
2].
Go to :

CASE
A 76-year-old male presents with jaundice and weight loss of 10 kg in 6 months. His past medical history was significant for systemic arterial hypertension. Initial evaluation outside our institute showed alterations in liver function tests characterized by cholestasis. An abdominal ultrasound revealed dilation of the extrahepatic bile duct up to 15 mm secondary to a 31 mm × 20 mm sized solid lesion in the pancreas head. The patient underwent a transendoscopic ultrasound with fine-needle aspiration (FNA) biopsy that revealed a malignant epithelial neoplasia compatible with PB and immunohistochemistry CK19 (+), P63(+), chromogranin A (–), synaptophysin (–), ACE (–), and Ki67 50%. The patient was referred to our institute to continue workup and treatment. Laboratory results revealed a total bilirubin level of 40.22 mg/dL, direct bilirubin level of 25.30 mg/dL, indirect bilirubin level of 14.92 mg/dL, alanine transaminase level of 104 U/L, aspartate transaminase level of 99 U/L, alkaline phosphatase level of 306.00 U/L, and albumin level of 3.9 g/dL. An abdominal computed tomography (CT) scan showed a 39-mm sized tumor in the pancreas head with loss of interface with the gastroduodenal artery and in close contact with the superior mesenteric vein (
Fig. 1). Additionally, slides reviewed from the previous FNA biopsy suggested PB without ruling out acinar cell carcinoma. The patient underwent pyloric-sparing pancreaticoduodenectomy without postoperative complications. Gross and microscopic pathological examinations demonstrated a 40 × 40 mm sized lesion compatible with adult-type PB. It was associated with lymphovascular and perineural invasion without lymph node metastases. It showed an immunohistochemical profile of chromogranin (–), synaptophysin (+), CK7 (+), and CK19 (+) (
Fig. 2). The patient was discharged home on postoperative day five and given an adjuvant chemotherapy with Capecitabine 2,000 mg/m
2 and cisplatin 60 mg/m
2 for three cycles. The patient is currently in follow-up. The latest follow-up showed no evidence of the disease at 32 months after his initial diagnosis.
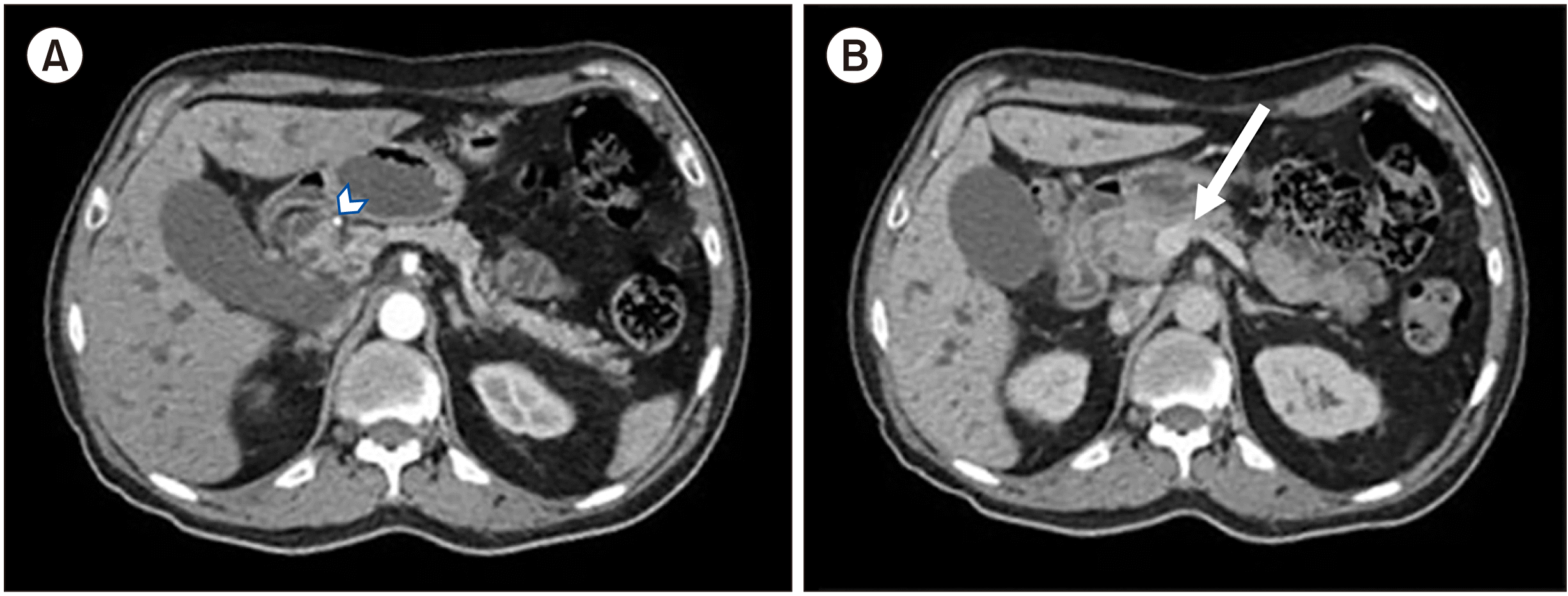 | Fig. 1Abdominal computed tomography scan showing a 3.6 cm × 2.3 cm × 3 9 cm sized lesion on the pancreas head that looks (A) hypodense in the arterial phase and (B) isodense to the parenchyma in the venous phase. It loses interface with the gastroduodenal artery (arrowhead) and the celiac trunk as well as superior mesenteric artery. However, it has contacts with the right medial border of the superior mesenteric vein (arrow) without producing stenosis or thrombosis. 
|
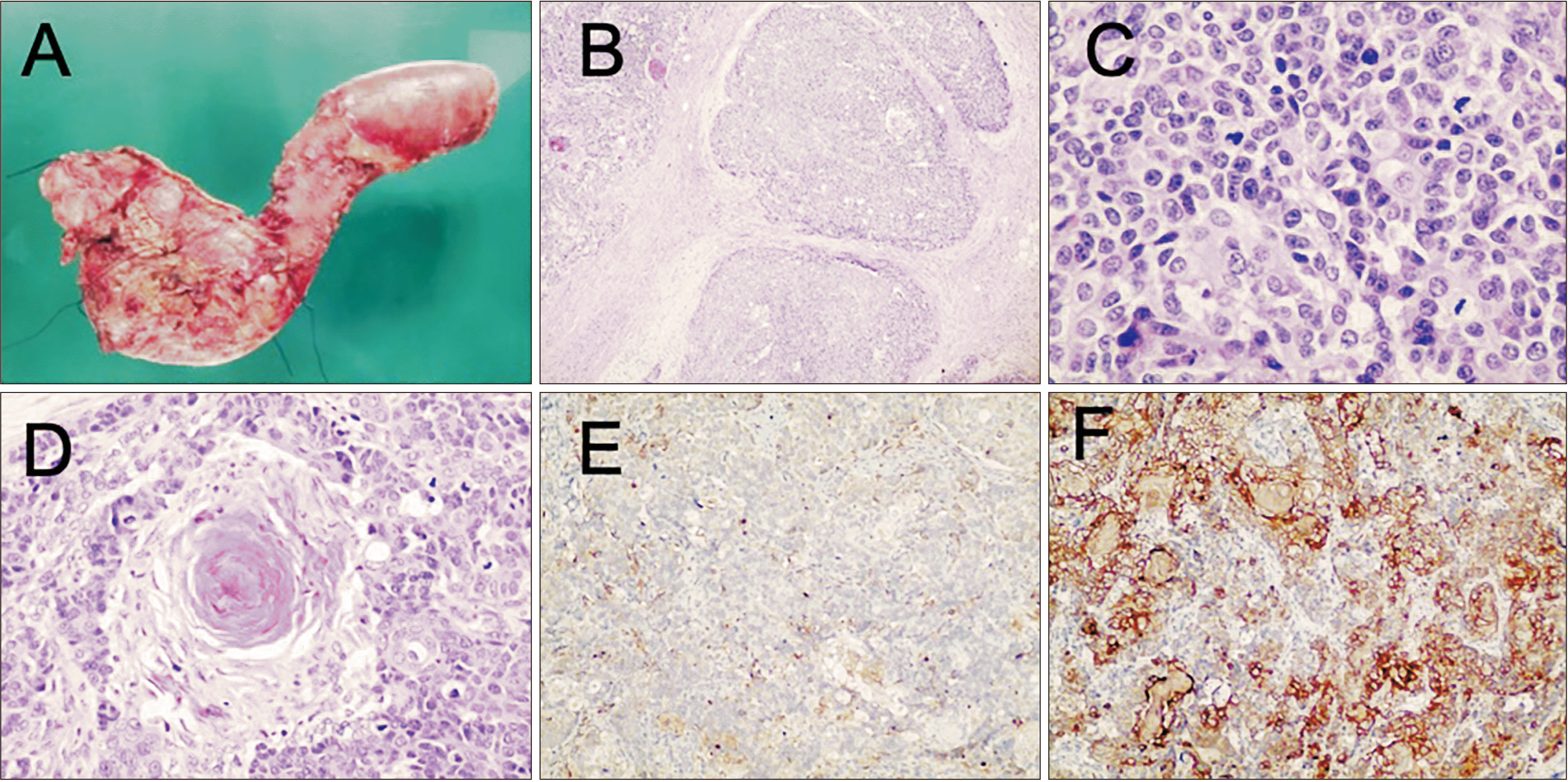 | Fig. 2Macroscopic evaluation (A) with smooth and yellowish lesion on the pancreatic head that presents irregular borders. Microscopic evaluation (B) showing highly cellular lobules separated by thick fibrous bands, simulating an acinar structure (H&E 4×). (C) Neoplastic cells showing an organoid arrangement around small lumina, with mild nuclear atypia, granular eosinophilic cytoplasm and abundant mitoses (H&E 40×). (D) Squamoid nests are considered as defining components, consisting of circumscribed whorled nests of plump spindle cells with a squamous appearance and keratinization (H&E 30 ×). Immunolabeling for (E) alpha1-antichymotrypsin (H&E 4×) and (F) cytokeratin 19 is highly sensible for this neoplasm (H&E 4×). 
|
Go to :

DISCUSSION
PB is an infrequent malignant neoplasm of the pancreas. It can present a diagnostic challenge. Abdominal pain and mass are the most frequent clinical manifestations, as is the case in our patient. It is rarely associated with jaundice and symptoms of gastrointestinal dysfunction. Its differential diagnosis includes poorly differentiated adenocarcinomas, solid pseudopapillary tumors, pancreatic carcinoma, pancreatic neuroendocrine tumors, and autoimmune pancreatitis, for which complementary studies are recommended [
3].
It can appear as a well-circumscribed and heterogeneous mass with a solid and cystic component [
4,
5] on ultrasound. Other forms of presentation may be cystic with internal septa and occasionally only solid component [
4,
6]. On tomography, the tumor may be totally or partially circumscribed [
6]. Its multilobed contours contain cystic areas, septations, and small hyperdense foci, some punctate and others curvilinear compatible with calcifications. It can be differentiated from other rare pancreatic tumors by imaging [
7].
Serum tumor markers such as carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) are neither consistent nor helpful in diagnosing adult PB.
Diagnosis with FNA is difficult due to multiple differentiation lines that resemble cytologic features seen in other tumors such as the pancreas’ acinar cell carcinoma. Thus, the definitive diagnosis of PB depends on the pathological examination characterized by the presence of squamous cell nests with prominent acinar differentiation and foci of ductal, squamous, and endocrine cells [
8,
9].
Immunohistochemical evaluation can show various cellular differentiation features: CEA and B72.3 for ductal differentiation, chromogranin and synaptophysin for the neuroendocrine component, and trypsin or chymotrypsin for acinar differentiation.
Currently, surgical resection is the recommended treatment associated with the best long-term survival [
10]. The criteria for adjuvant chemotherapy are not well established yet due to the small series reported in the literature. Some authors recommend chemotherapy only for patients with recurrent, residual, or irresectable disease. However, because of its highly metastatic potential, other authors advocate for aggressive treatment with adjuvant therapy in all cases [
11].
This case represents the most infrequent scenario of a male patient in the eighth decade of life with a lesion in the head of the pancreas having 10 months of evolution. During work up, malignant cells were observed on microcopy compatible with PB. Diagnosis was made after pathologic examination showing the presence of multiple lines of differentiation resembling fetal pancreas tissue. Surgical resection with negative margins is the first-line treatment. Resectability of the tumor, functional status of the patient, and life expectancy of the patient should always be taken into account when performing a surgical resection. Although our patient was 76-year-old, the risk-benefit analysis favored a surgical procedure to improve cholestasis and provide a period of progression-free survival. Overall survival is 15 months for patients operated exclusively and 20.5 months for patients treated with cycles of chemotherapy and surgery. Our patient’s 32-month survival is much higher than that described in the literature.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download