INTRODUCTION
CASE
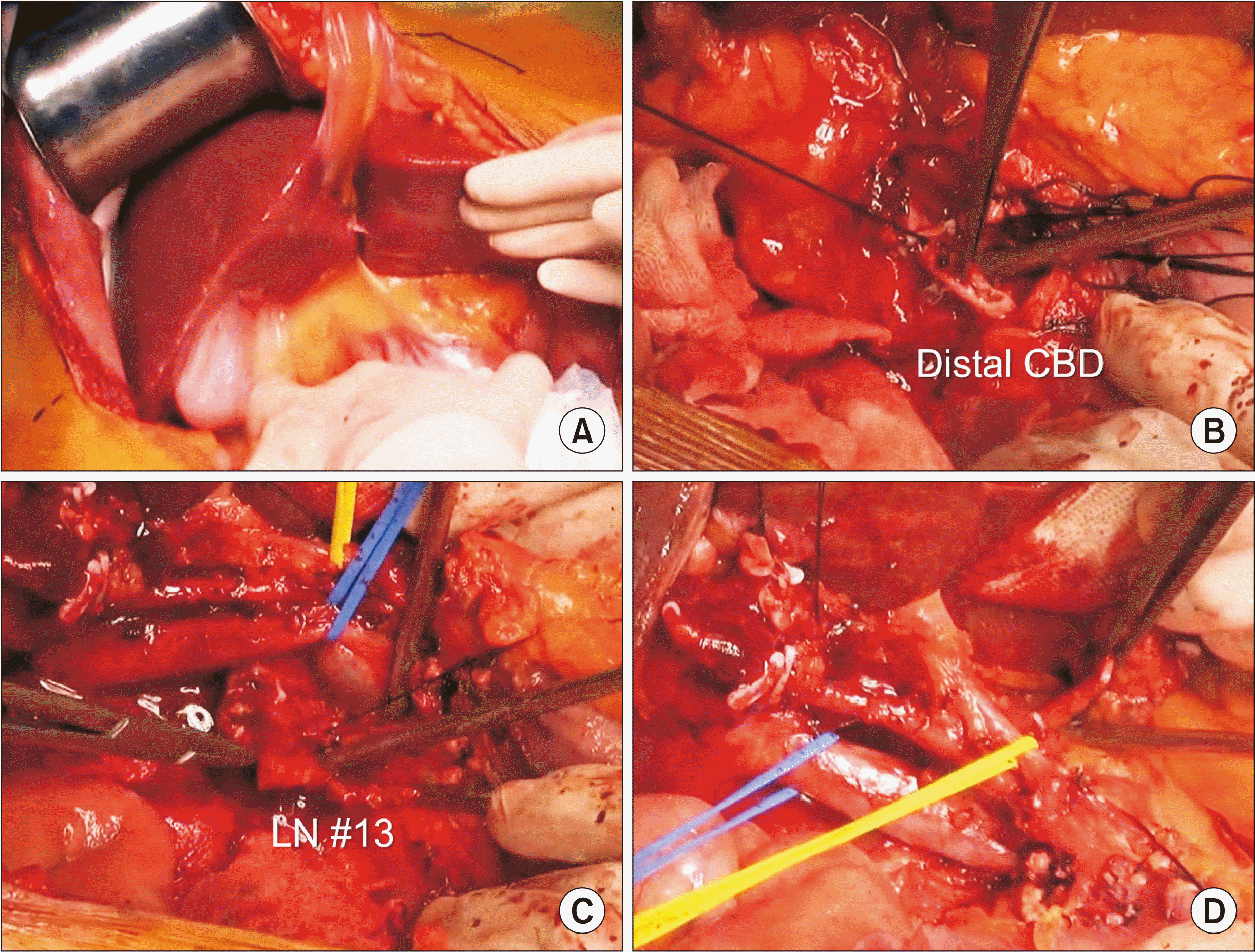 | Fig. 2Intraoperative photographs showing dissection of the hepatoduodenal ligament. Surgical resectability is assessed through manual palpation (A). The distal common bile duct (CBD) is transected (B). Enlarged regional lymph nodes (LNs) are dissected (C). Intraoperative frozen-section biopsy showed nodal metastasis. The hepatic artery and portal vein branches are isolated without macroscopic vascular invasion (D). |
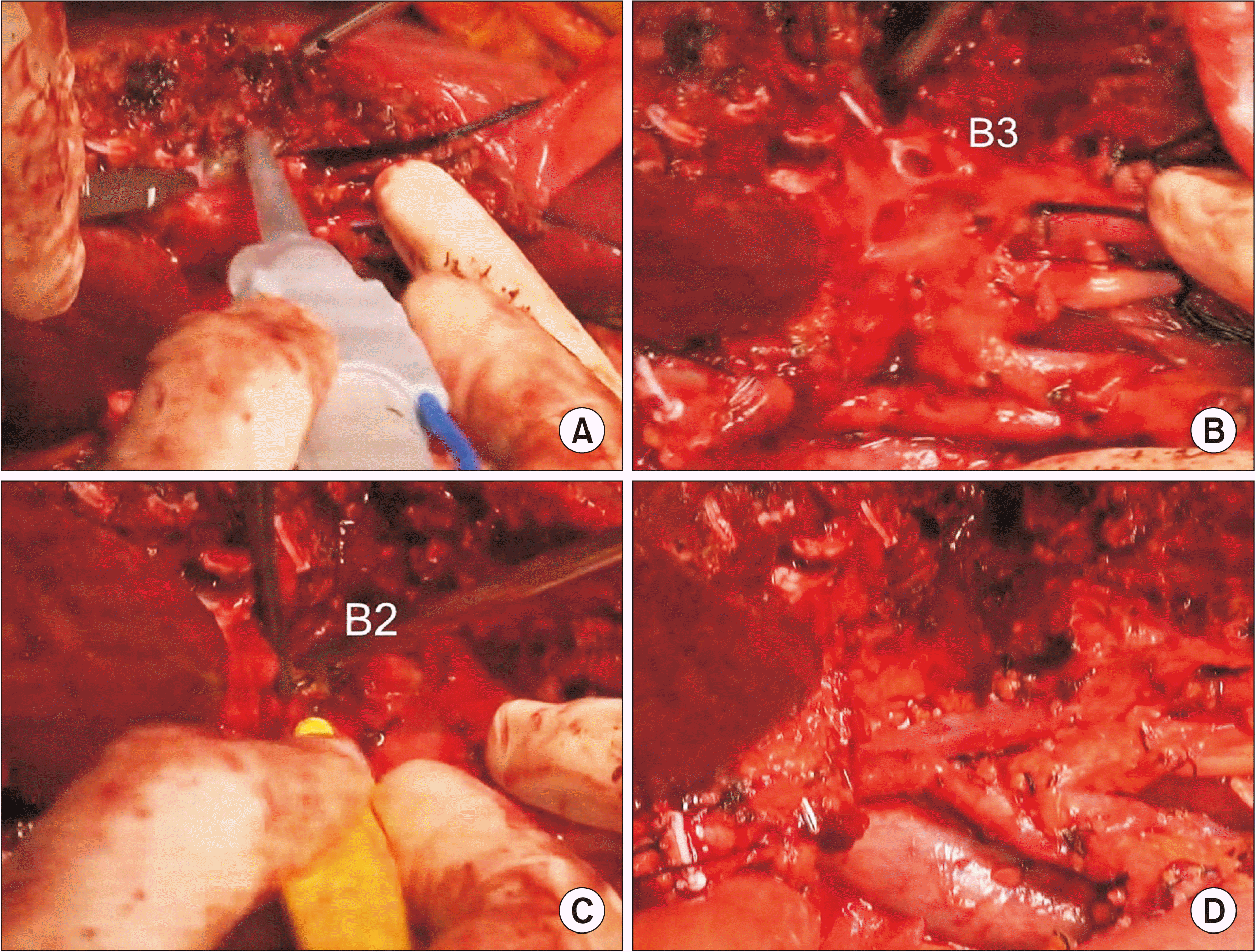 | Fig. 3Intraoperative photographs showing left liver transection. The liver parenchyma is transected along the falciform ligament (A). The segment III duct (B3) and segment II duct (B2) are consecutively transected (B, C). The left portal vein and hepatic artery are further dissected to expose the umbilical portion of the left portal vein, and the remnant left lateral segment is separated from the left caudate lobe (D). |
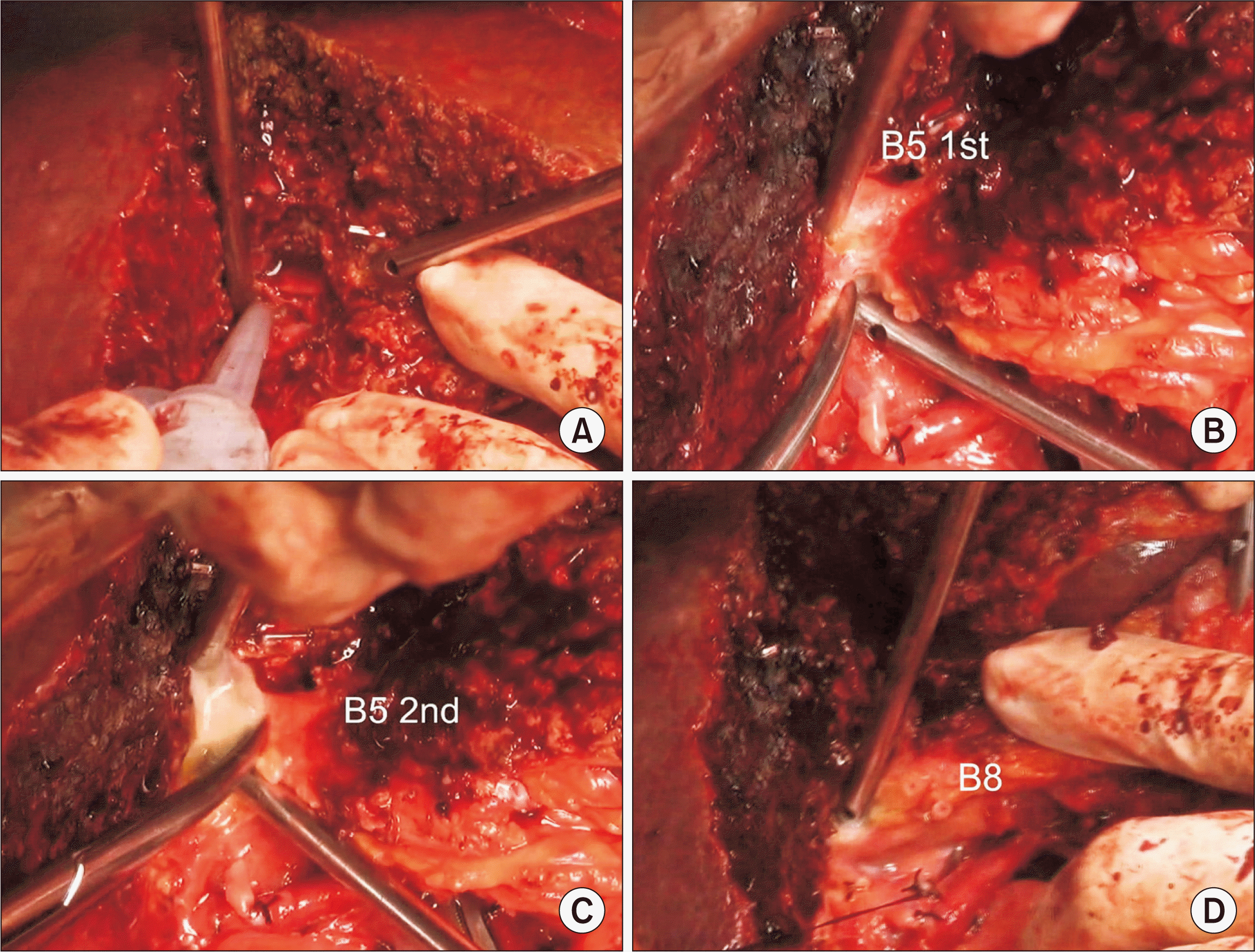 | Fig. 4Intraoperative photographs showing right liver transection. Right-sided hepatic parenchymal transection is performed along the hemi-liver discoloration line (A). Two segment V ducts (B5) are transected (B, C). Segment VIII duct (B8) is transected (D). |
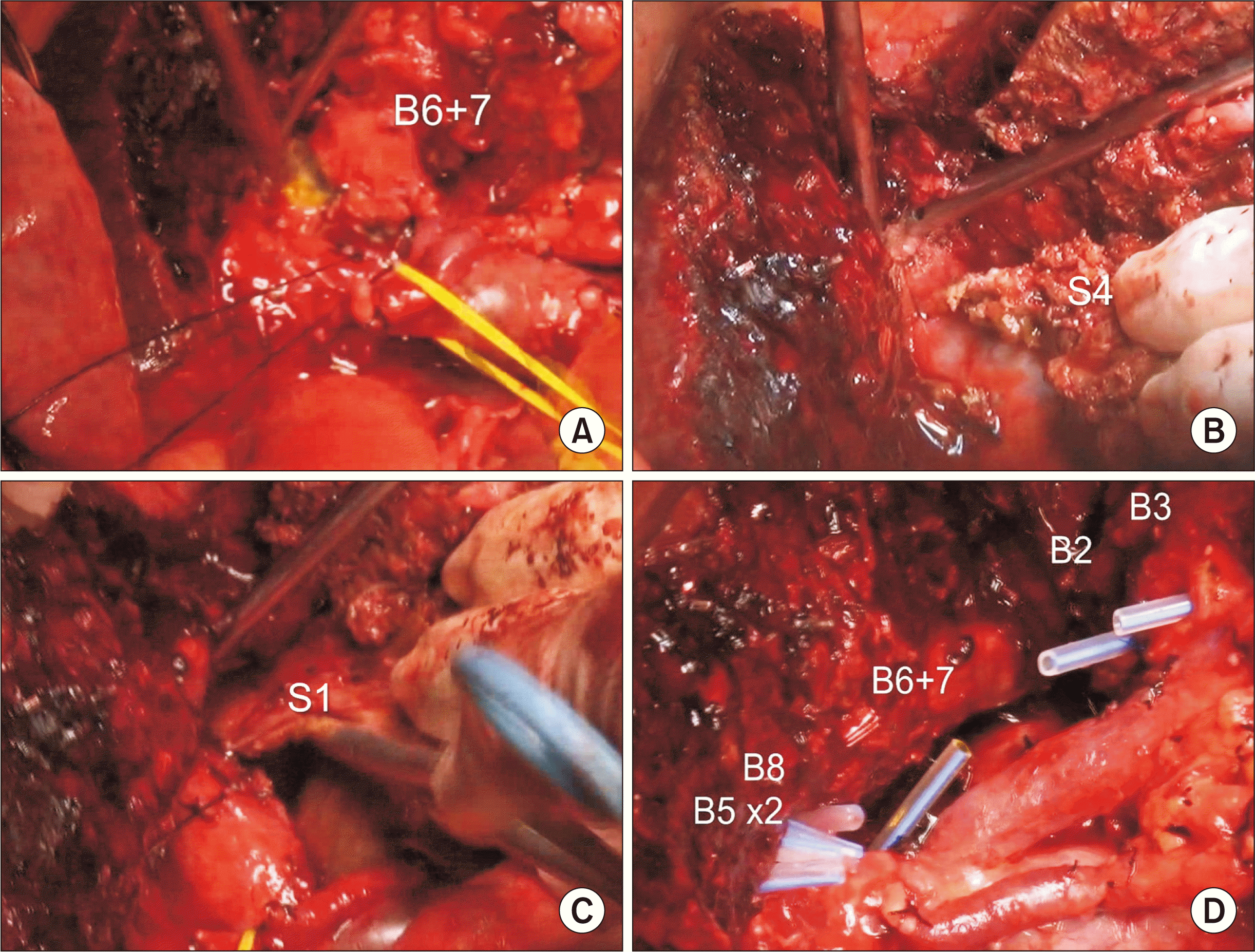 | Fig. 5Intraoperative photographs showing right liver transection. Conjoined segments VI and VII duct (B6 + 7) is transected (A). The paracaval portion and the left caudate lobe (S1) are removed along with segment IV (S4) resection (B, C). There are six hepatic duct openings at the left and right hila (D). |
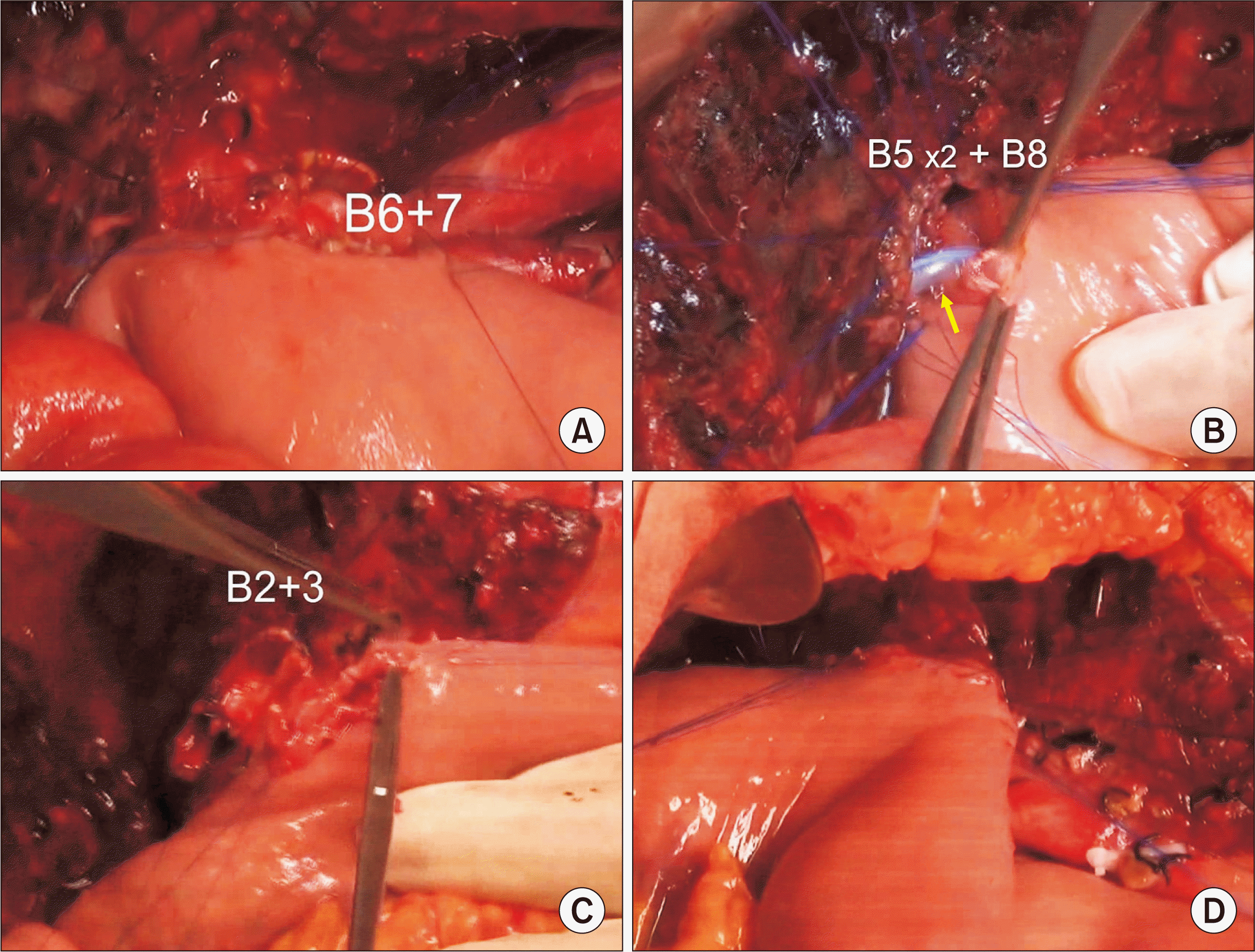 | Fig. 6Intraoperative photographs showing biliary reconstruction. Segments VI and VII duct (B6 + 7) is reconstructed through a single hepaticojejunostomy (HJ) (A). Two segment V ducts (B5) and one segment VIII duct (B8) are conjoined and a single HJ is performed with stent insertion into the small B5 duct (arrow) (B). Segment II and III ducts (B2 + 3) are conjoined and a single HJ is performed (C, D). |




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download