This article has been
cited by other articles in ScienceCentral.
Abstract
The left lateral section (LLS) can have an unusual variant left hepatic vein (LHV) anatomy. We present a case of customized funneling venoplasty of the graft LHV in a 22-month-old girl diagnosed with ornithine transcarbamylase deficiency undergoing deceased donor liver transplantation (LT) using a split LLS graft. The split LLS graft weighed 350 g, yielding a graft-to-recipient weight ratio of 3.2%. Notably, the graft LHV opening was located at the graft liver cut surface, which was only 1 cm in size and 2 cm away from the cephalad apex of the LLS graft. Since such a variant location of the small LHV opening was unsuitable for direct anastomosis, we performed a funneling venoplasty using an inferior vena cava fragment homograft obtained from the same donor. The graft implantation was performed according to standard procedures of infant split LT. Follow-up imaging studies showed no vascular complications. The patient recovered uneventfully from the LT operation. She had normal blood test findings, including normal ammonia level. She has been doing well for 6 months after the transplantation. In conclusion, our surgical technique using a funneling venoplasty enabled successful reconstruction of the anomalous graft LHV. Our results suggest that individualized reconstruction techniques should be applied to infant patients undergoing LT using a LLS graft with variant types of graft LHV anatomy.
Go to :

Keywords: Left hepatic vein, Anatomical variation, Venoplasty, Interposition, Left lateral section graft
INTRODUCTION
A left lateral section (LLS) graft is often used for liver transplantation (LT) in infant patients in the form of living donor liver transplantation (LDLT) or split LT. Although the anatomy of the left hepatic vein (LHV) is diverse [
1-
4], the majority of graft LHVs are suitable for direct anastomosis to the recipient hepatic vein stumps or directly to the inferior vena cava (IVC). However, in rare instances, the LLS outflow drains through both the LHV and the middle hepatic vein (MHV) or the LHV drains directly through the MHV trunk [
1]. In donors with such unusual LHV anatomy, it is necessary to preserve the MHV trunk for the safety of the living donor or for the security of the split extended right liver graft. If the graft LHV opening is located at the cut surface of the LLS graft instead of at the cephalic apex, a customized venoplasty technique is necessary to make it suitable for graft hepatic vein reconstruction, especially for an infant recipient with a small IVC. We present a case of customized venoplasty of the graft LHV in an infant patient undergoing deceased donor LT using a split LLS graft.
Go to :

CASE
The recipient was a 22-month-old girl who was diagnosed with ornithine transcarbamylase (OTC) deficiency. The patient was born through a full-term cesarean-section delivery. She showed irritability and decreased activity from one month after birth. At that time, laboratory studies showed hyperammonemia and metabolic acidosis with high levels of liver enzymes. Gene studies revealed OTC NM_000531.5:c.626C>T. p.Ala209V. Het. The patient was placed on the waiting list of the Korean Network for Organ Sharing (KONOS) with a Pediatric End-stage Liver Disease score of 4 (total bilirubin level of 0.6 mg/dL, albumin level of 4.4 g/dL, prothrombin international normalized ratio of 1.37, and growth failure).
After a waiting period of one year, a 35-year-old female deceased donor was allocated for split LT. At organ allocation, the patient’s height and body weight were 85 cm and 11 kg, respectively. The split LLS graft weighed 350 g, yielding a graft-to-recipient weight ratio (GRWR) of 3.2%.
Notably, the graft LHV opening was located at the graft liver cut surface, which was only 1 cm in size and 2 cm away from the cephalad apex of the LLS graft. The native anatomy of the donor LHV was presumed to be type 3 (
Fig. 1,
2) [
1]. Since such a variant location of the small LHV opening was unsuitable for direct anastomosis, we performed a funneling venoplasty using an IVC fragment homograft obtained from the same donor (
Fig. 3,
4).
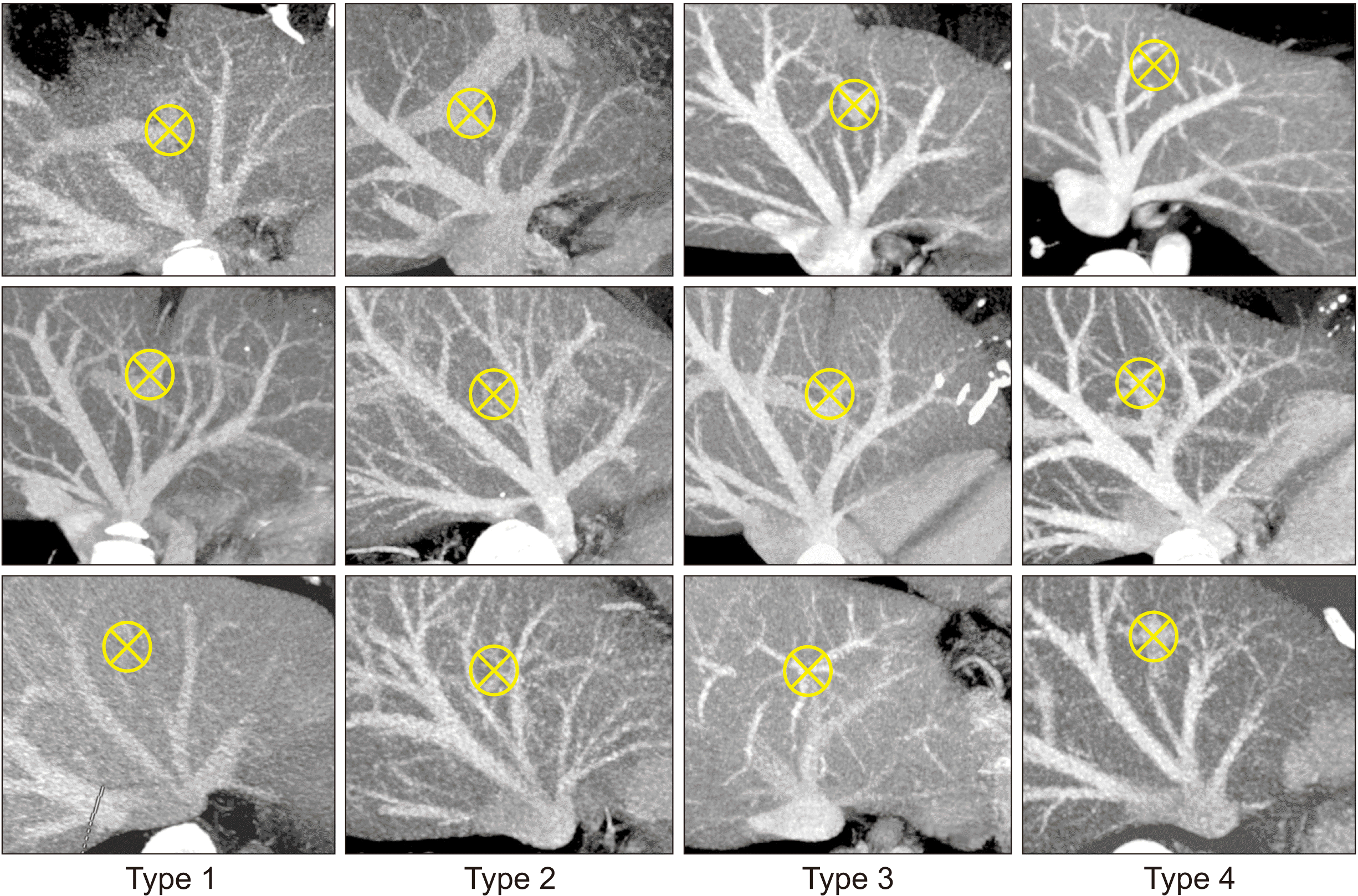 | Fig. 1Classification of the hepatic vein anatomy in the left lateral section in terms of patterns of the left lateral section graft hepatic vein openings. Type 1 makes a single opening. Type 2 makes two widely spaced openings. Type 3 makes large and small adjacent openings. Type 4 makes two widely spaced openings. Crossed circles indicate the location of the umbilical portion. Cited from the article of Hwang et al. (Liver Transpl 2013;19:184-190) [ 1]. 
|
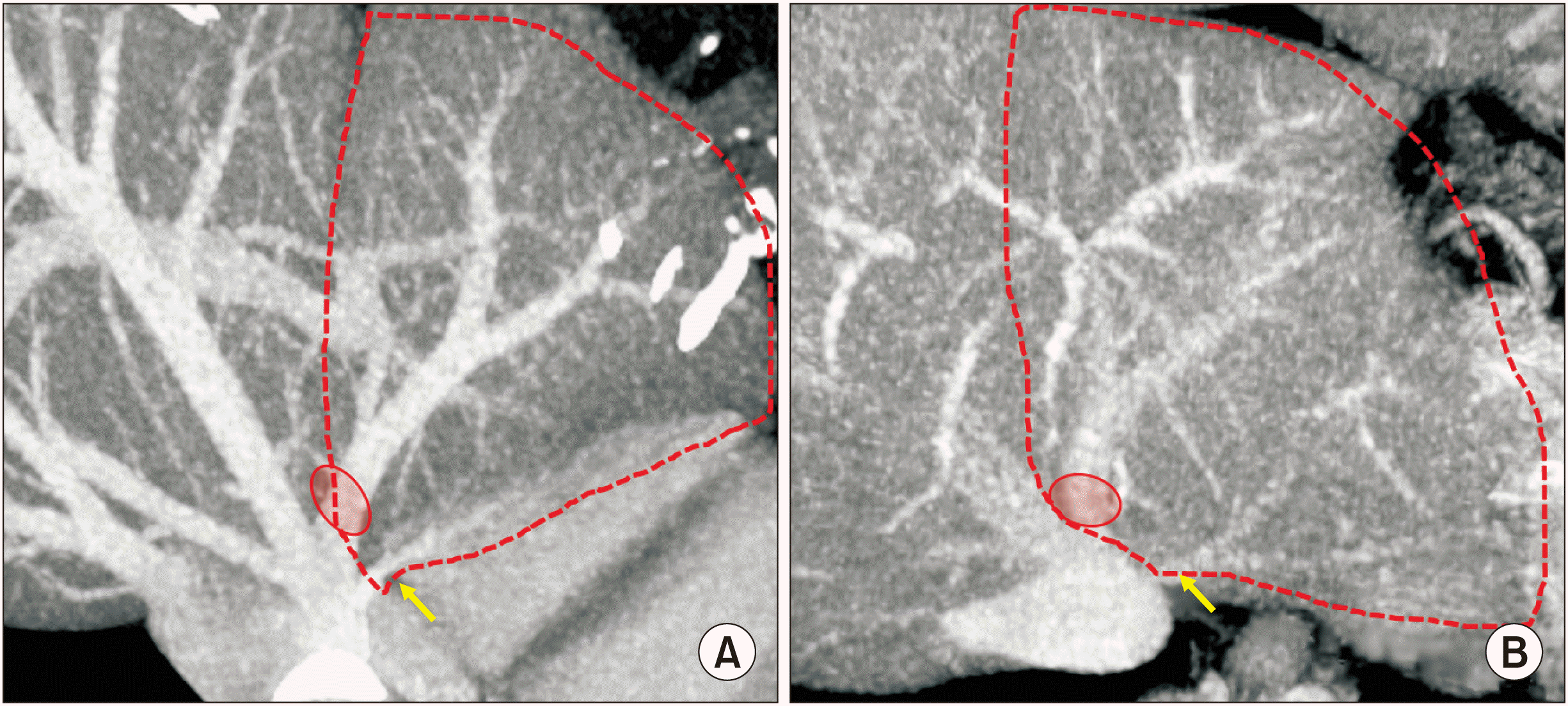 | Fig. 2Presumed anatomy of the donor liver. The anatomy of the left hepatic vein appears to be a mixed type of two images with narrow (A) and wide (B) distances between the large and small hepatic vein openings. The small opening indicates the superficial branch of the left hepatic vein (arrows). 
|
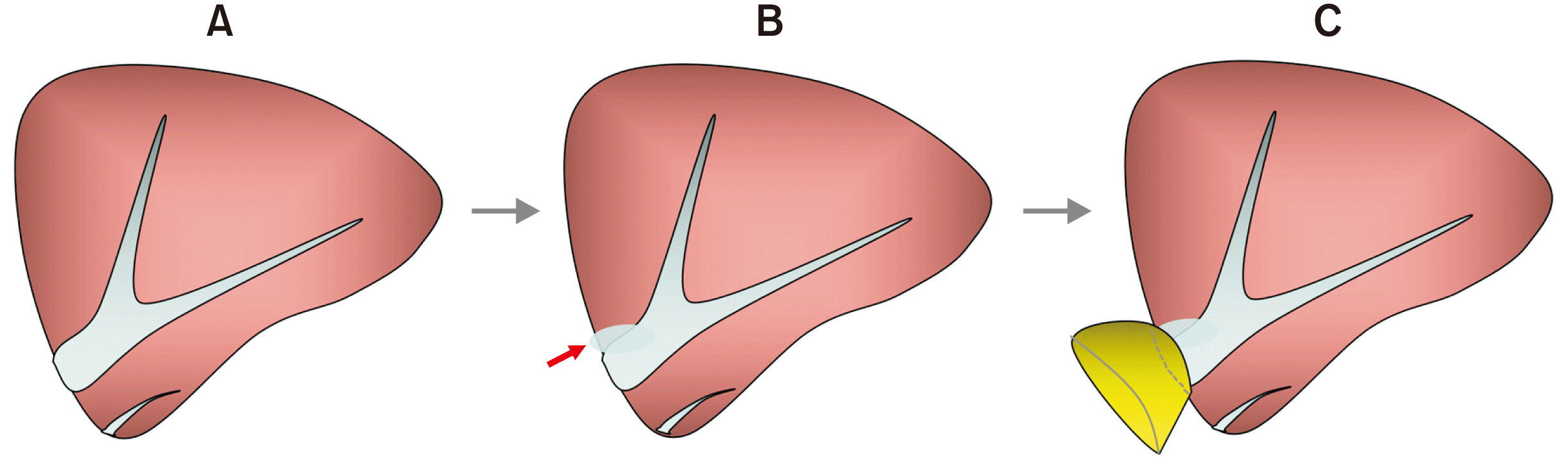 | Fig. 3Design of customized funneling venoplasty for the graft left hepatic vein opening. The 1 cm-sized orifice (A) is partially incised to increase the diameter (B). A vein patch is attached at the enlarged graft hepatic vein opening to make a funnel-shaped conduit (C). Arrow indicates a slit incision. 
|
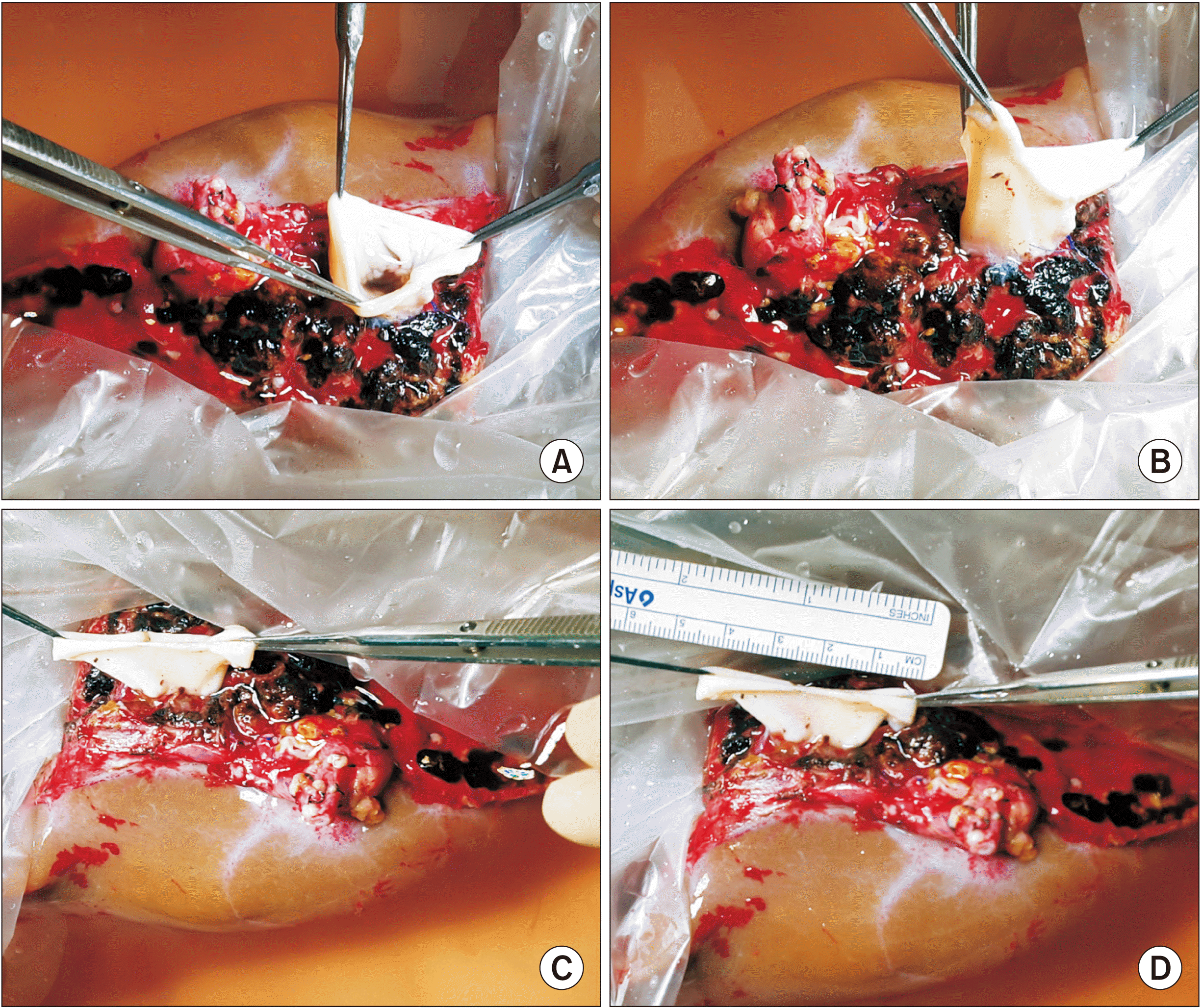 | Fig. 4Intraoperative photographs of bench work. (A–C) An inferior vena cava fragment homograft is attached to the graft hepatic vein orifice to make a funnel-shaped conduit. (D) The cephalad end of the conduit is much larger than the graft side. 
|
Because there was no anatomical variation in the recipient (
Fig. 5), standard procedures of pediatric split LT were performed. After dissection of the recipient native liver was completed, the hepatic parenchyma was incised with a surgical knife, leaving a bulk of hepatic parenchyma around the hepatic vein trunks. The hepatic parenchyma was forcefully pulled out to detach it from the hepatic vein stumps, which made stump walls long and thick. No venoplasty was applied to the recipient IVC orifice. The interposed funnel-shaped orifice of the graft hepatic vein was anastomosed with the recipient IVC orifice by 1 : 1 size matching (
Fig. 6). The recipient portal vein was normal-looking, thus it was anastomosed with the graft portal vein stump using a branch patch (
Fig. 7). The graft hepatic artery was reconstructed under surgical microscopy. Finally, Roux-en-Y hepaticojejunostomy was performed.
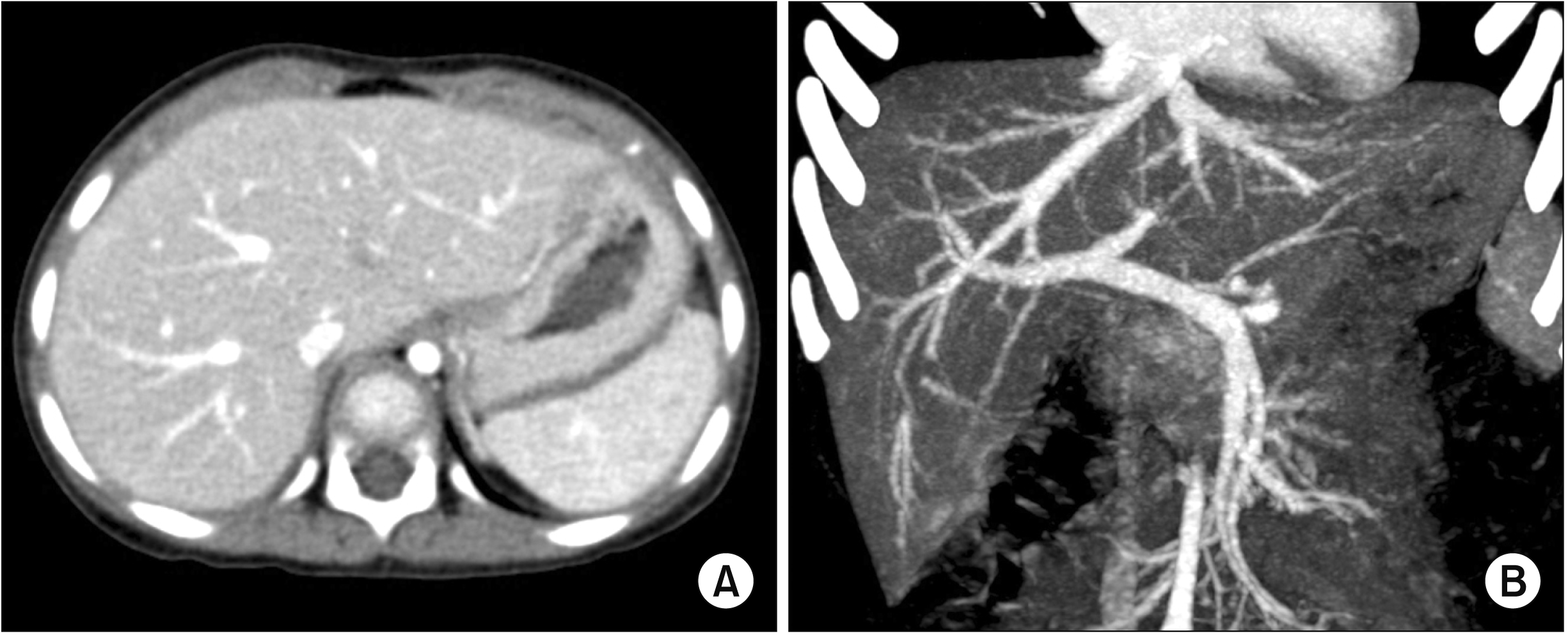 | Fig. 5Preoperative computed tomography images showing no gross abnormality in liver shape (A) or hepatic vasculature (B). 
|
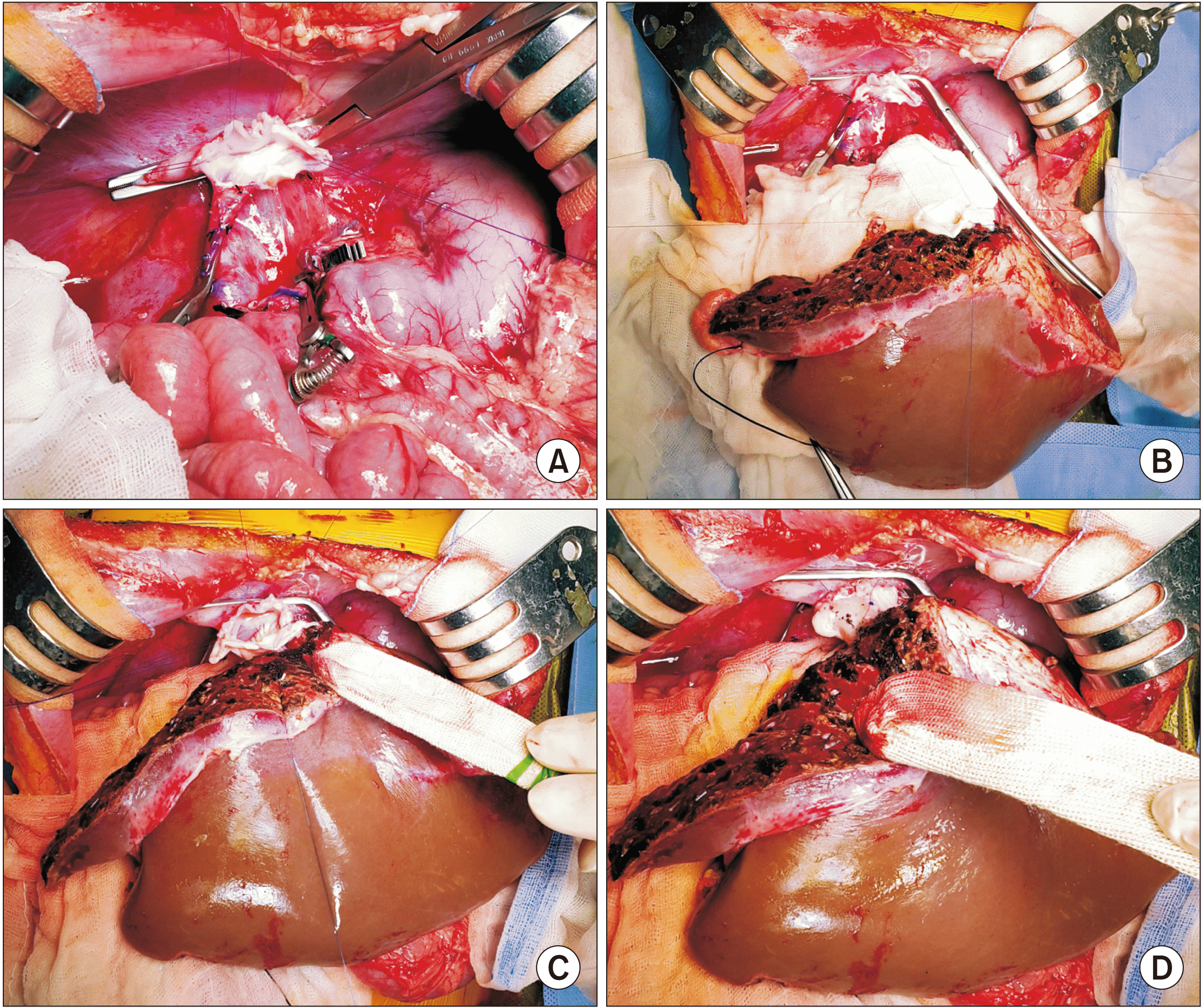 | Fig. 6Intraoperative photographs of recipient hepatic vein reconstruction. (A) The three hepatic vein openings are widely opened to make an enlarged orifice. (B) The hepatic vein openings at the recipient inferior vena cava and the graft are well matched in size. (C) The posterior wall of the hepatic vein reconstruction is visible. (D) The anterior wall of the hepatic vein reconstruction is visible. 
|
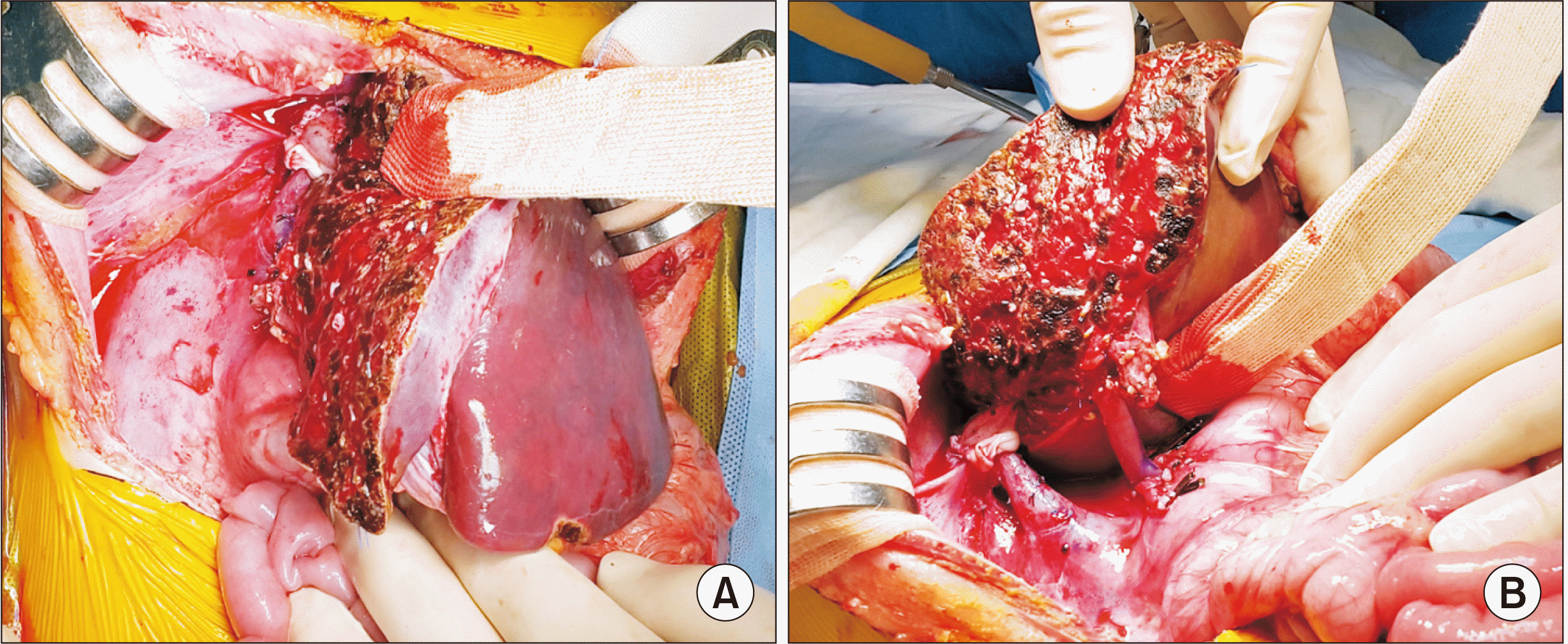 | Fig. 7Intraoperative photographs of left lateral section graft implantation. (A) The hepatic vein reconstruction is located at the orthodox position. (B) Portal vein reconstruction is performed using a branch patch of the recipient portal vein. 
|
The pathology report of the explant liver revealed enlarged hepatocytes with pale cytoplasm and halo nuclei, and multifocal aggregates of apoptotic cells, consistent with OTC (
Fig. 8). Early follow-up computed tomography scan showed no vascular complications (
Fig. 9). The patient recovered uneventfully from the LT operation. She had normal blood test findings including normal ammonia level. She has been doing well for six months after the transplantation.
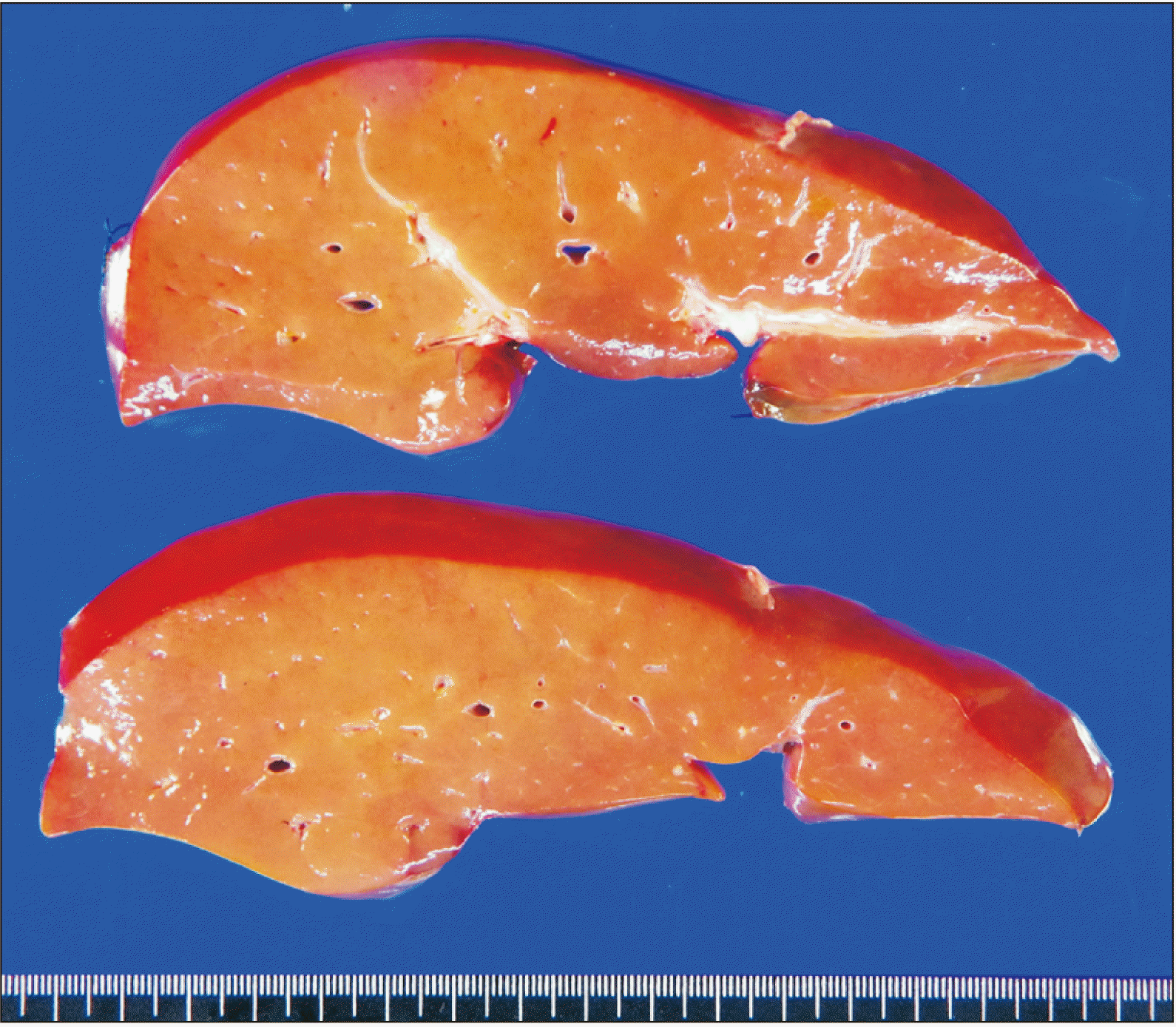 | Fig. 8Gross photograph of the explant liver. 
|
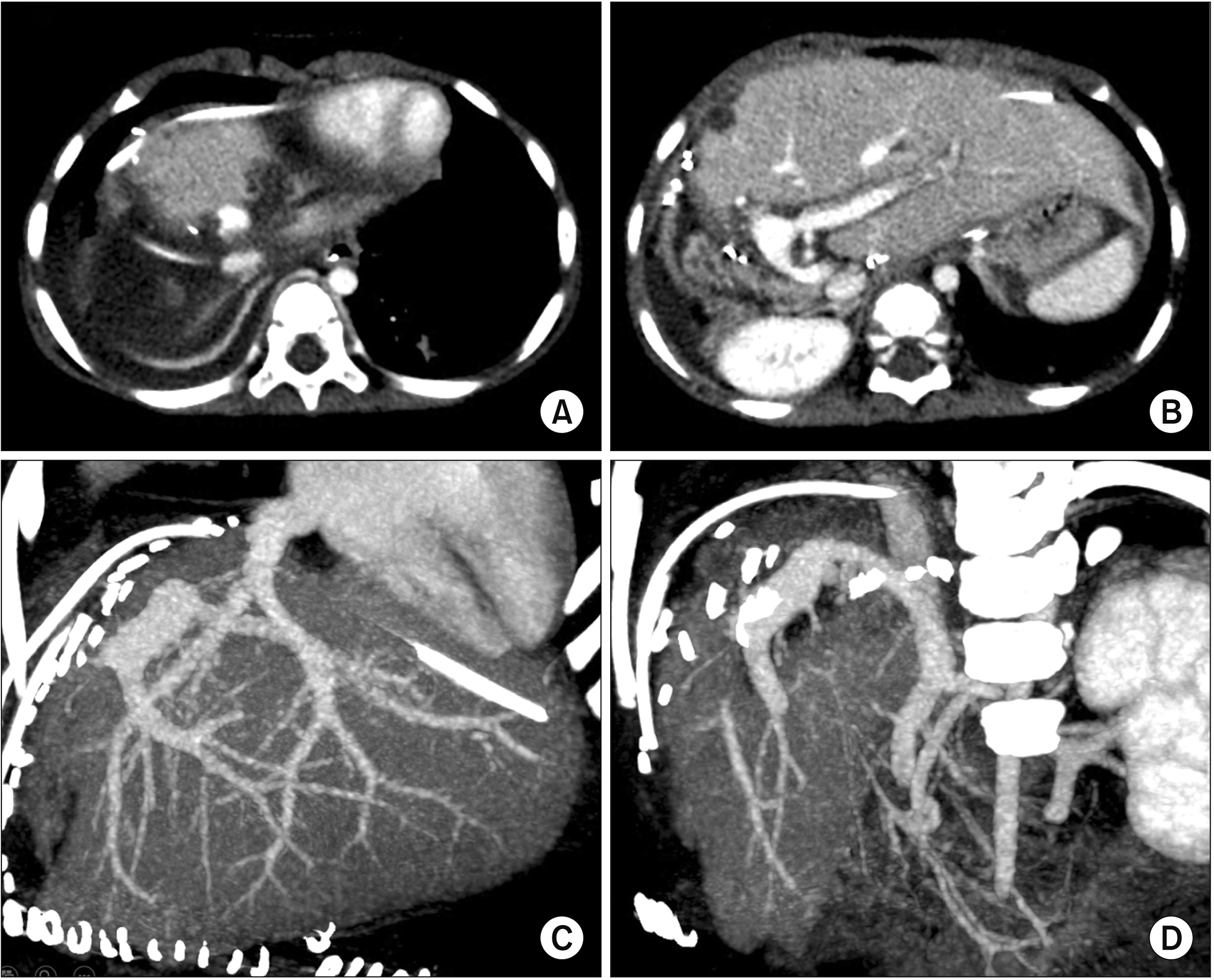 | Fig. 9Follow-up computed tomography images taken 4 days after liver transplantation. The hepatic vein reconstruction (A, C) appears smooth and streamlined without stenosis. The portal vein reconstruction (B, D) shows a size discrepancy without noticeable stenosis. 
|
Go to :

DISCUSSION
LLS grafts rarely have LHV variations of sufficient significance to preclude direct reconstruction because a variant LHV can join with the MHV trunk in the left medial section. Because the left medial section parenchyma is sacrificed during LLS graft procurement [
5,
6], the LHV branches in the left medial section can be harvested to obtain a single graft hepatic vein orifice. However, infant recipients usually require small LLS grafts to adequately match the GRWR. Thus, LLS grafts usually only have the LHV trunk. If the graft LHV orifice is unsuitable for direct anastomosis, it is necessary to use customized venoplasty of the graft LHV to prevent graft hepatic vein outflow obstruction (HVOO) [
1,
7].
We have previously classified the LHV anatomy of 300 potential LLS graft donors into four types according to the number and location of graft LHV openings: single opening (type 1; n = 218, 72.7%), two large adjacent openings (type 2; n = 29, 9.7%), one large and one small adjacent opening (type 3; n = 34, 11.3%), and two widely spaced openings (type 4; n = 19, 6.3%) (
Fig. 1). LHV types 2 and 3 require wedged unification venoplasty and type 4 requires additional vein interposition [
1]. The LHV anatomy of the present LLS graft can be classified as a type 3, in which one large LHV branch is drained directly through the MHV trunk with the presence of a small superficial LHV branch. We had thought that this superficial LHV branch could be used for unification venoplasty of the LHV opening [
7]. However, we had to sacrifice it in the present case because it was widely apart from the LHV trunk with a size less than 2 mm.
In our previous LDLT case using a LLS graft with type 4 LHV, customized interposition-wedged unification venoplasty was used and the source of the interposition vein conduit was an ilio-femoral vein homograft [
1]. In the present case, because the required length of the vein conduit was 2 cm and the diameter of the graft LHV opening was only 1 cm, we thought that direct anastomosis might have a high risk of graft HVOO. To cope with the anatomical variation, we made a funneling venoplasty using an IVC fragment homograft patch to connect the recipient IVC orifice and the graft LHV orifice. The final configuration of the graft hepatic vein reconstruction appeared natural and stream-lined, as in an LLS graft with type 1 LLV anatomy.
LDLT or split LT for infant recipients is vulnerable to vascular complications because the graft and recipient vessels are much smaller than those in adult-to-adult LT. Anastomotic stenosis following hepatic vein reconstruction using an LLS graft is usually attributed to the small size of vascular anastomosis. Once HVOO develops, it is often difficult to treat it through radiologic angioplasty [
8-
11]. Insertion of a wall stent to treat HVOO is considered a life-saving procedure, with a high likelihood of the need for retransplantation later because such a vascular wall stent may not expand sufficiently during physical growth of the recipient from infancy to adolescence [
12,
13]. Therefore, graft hepatic vein reconstruction requires a secure surgical design, particularly in infant recipients.
In conclusion, our surgical technique using funneling venoplasty enabled successful reconstruction of the anomalous graft LHV. Our results suggest that individualized reconstruction techniques should be applied for infant patients undergoing LT using a LLS graft with variant types of graft LHV.
Go to :










 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download