INTRODUCTION
CASE
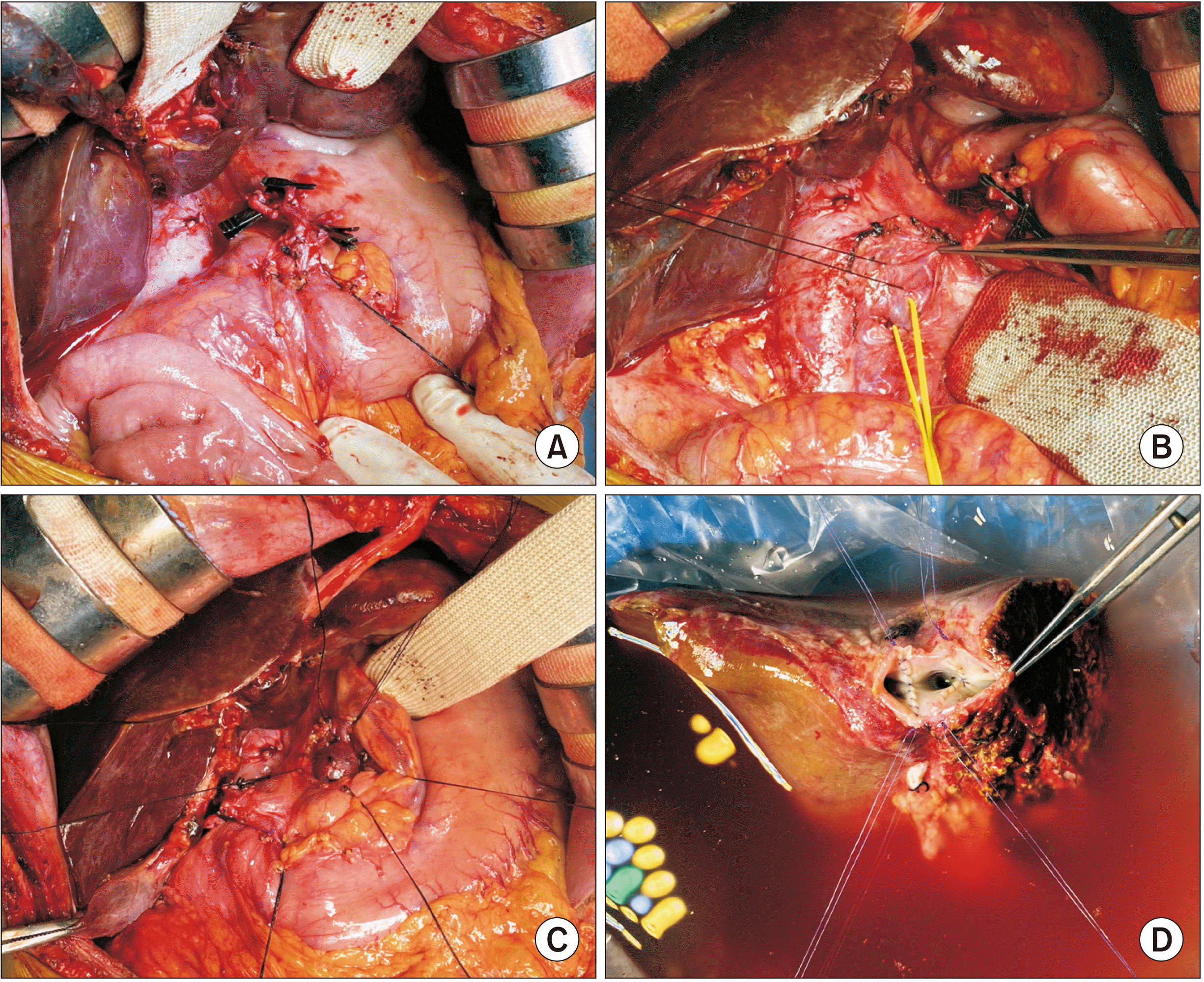 | Fig. 3Intraoperative photographs for recipient hepatectomy and graft bench work. (A) The hepatic artery branches are meticulously dissected after transection of the common bile duct. (B) The retropancreatic space is dissected and the left renal vein (yellow vessel loop) and the insertion site of the splenorenal shunt (black silk sling) are isolated. (C) The confluence portion of the mesentero-splenic vein is meticulously dissected. (D) The graft hepatic veins are separated into three openings, which are unified to make a wide single orifice. |
 | Fig. 4Intraoperative photographs of the hepatic vein and portal vein venoplasty. (A) The hepatic vein orifices at the recipient inferior vena cava are unified with venoplasty using a cryopreserved saphenous vein patch. (B) The vein branches at the mesentero-splenic confluence portion are securely clamped. (C) A 1.5 cm-long longitudinal incision is made at the confluence portion. (D–F) A 4 cm-long fresh-stored iliac vein conduit is anastomosed to the confluence portion in an end-to-side fashion. |
 | Fig. 5Intraoperative photographs of graft implantation. (A, B) The graft hepatic vein orifice is anastomosed with the size-matched recipient hepatic vein orifice. (C, D) The portal iliac vein conduit is anastomosed with the graft portal vein. (E) The congenital splenorenal shunt is securely ligated. (F) Portal blood flow is increased after ligation of the splenorenal shunt. |
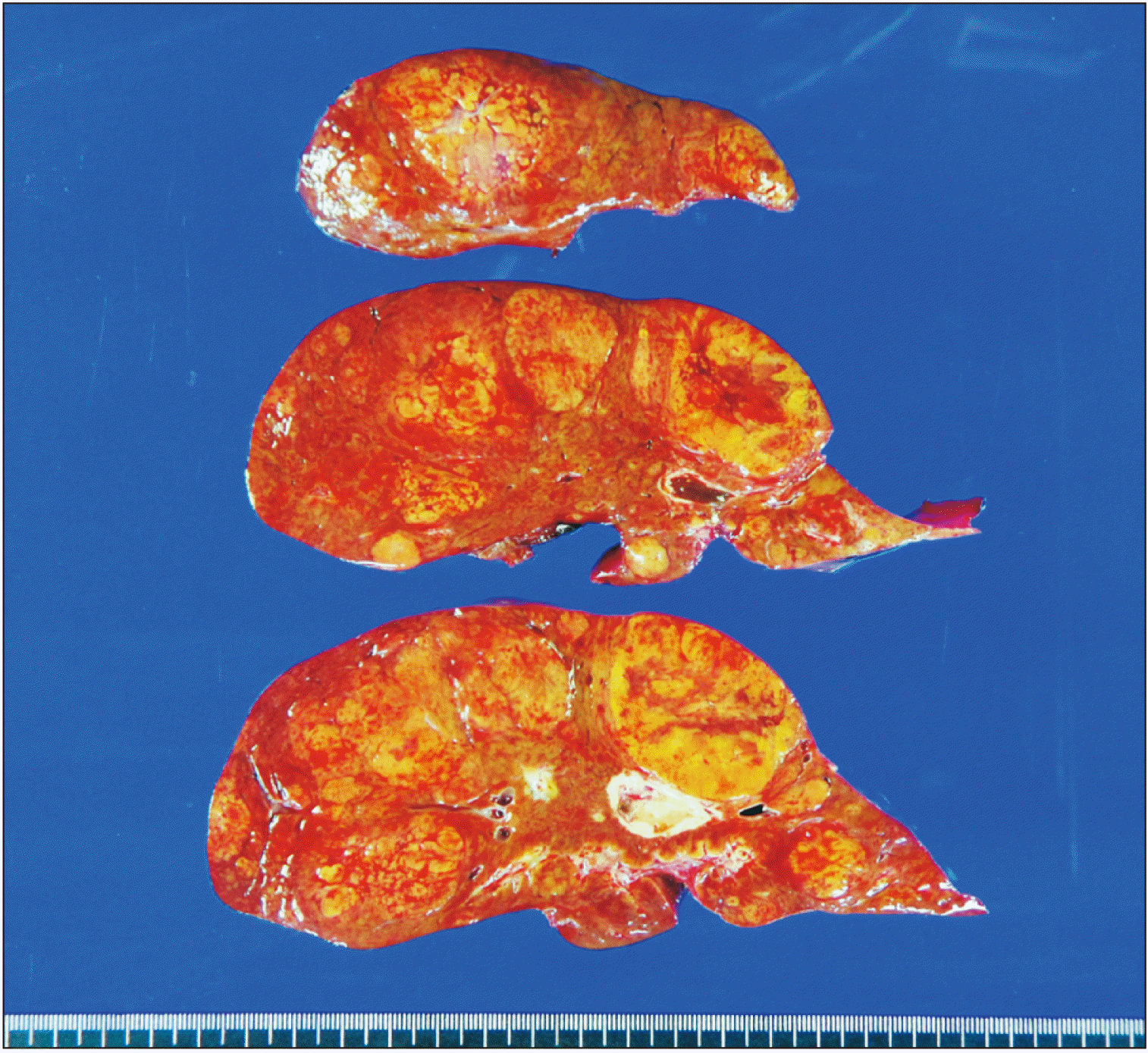 | Fig. 7Gross photographs of the explant liver. There are three types of liver nodules. A 2 cm-sized hepatocellular adenoma of beta-catenin mutated subtype is located at segment IV. Multiple focal nodular hyperplasia nodules measuring up to 7.1 cm in size are scattered over both lobes. Multiple regenerative nodules measuring up to 2.7 cm in size are also present in both lobes. |
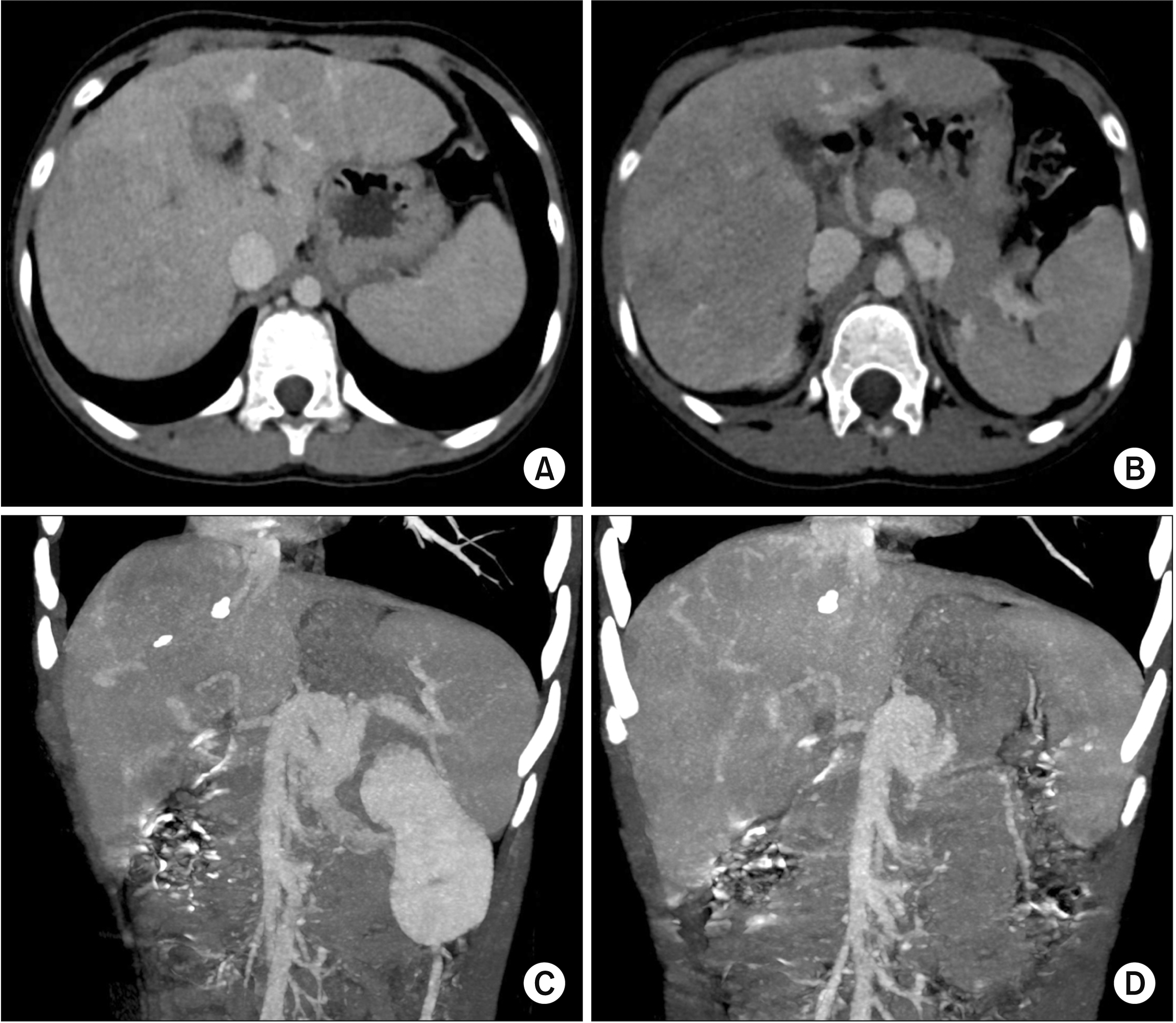
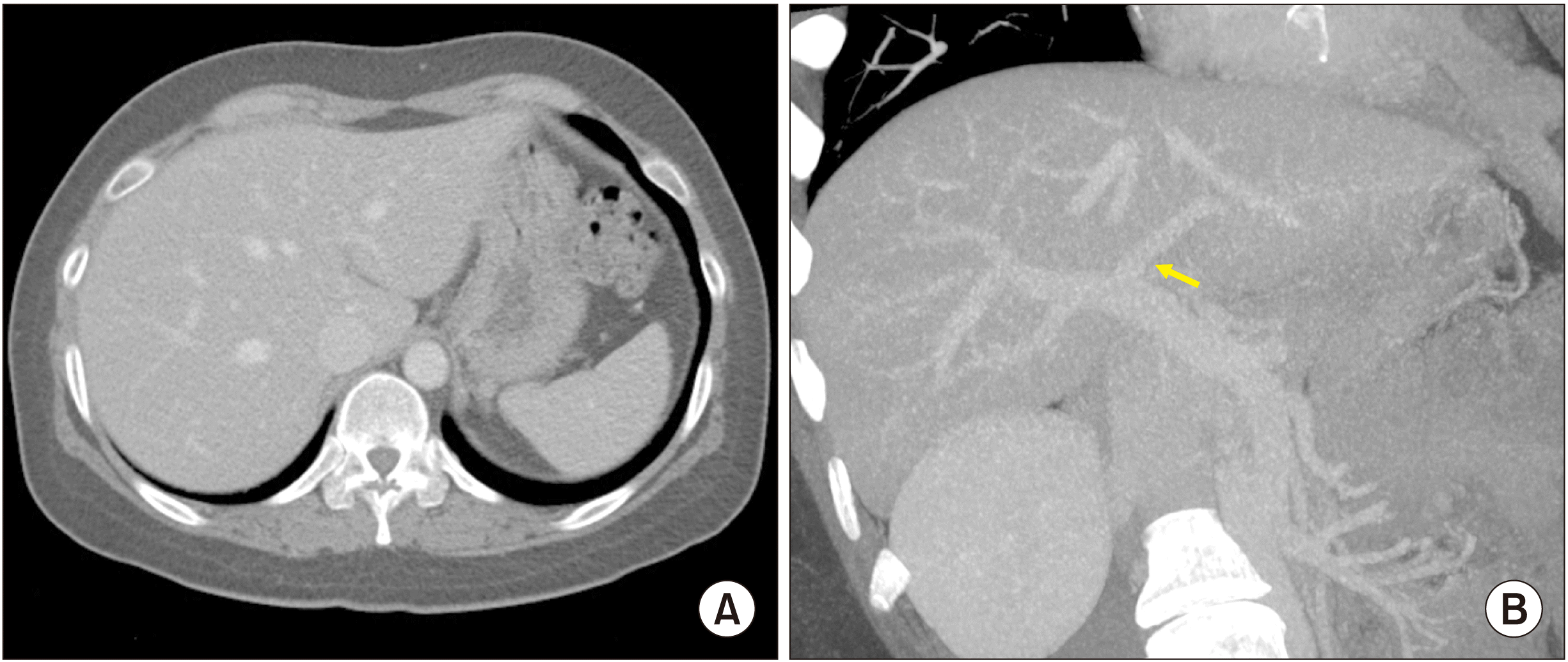
 | Fig. 8Posttransplant computed tomography scan taken at four days after the transplantation. (A, B) The graft portal vein reconstruction appears smooth and streamlined. (C, D) The iliac vein conduit from the mesentero-splenic vein junction is well visualized as a newly made main portal vein. |
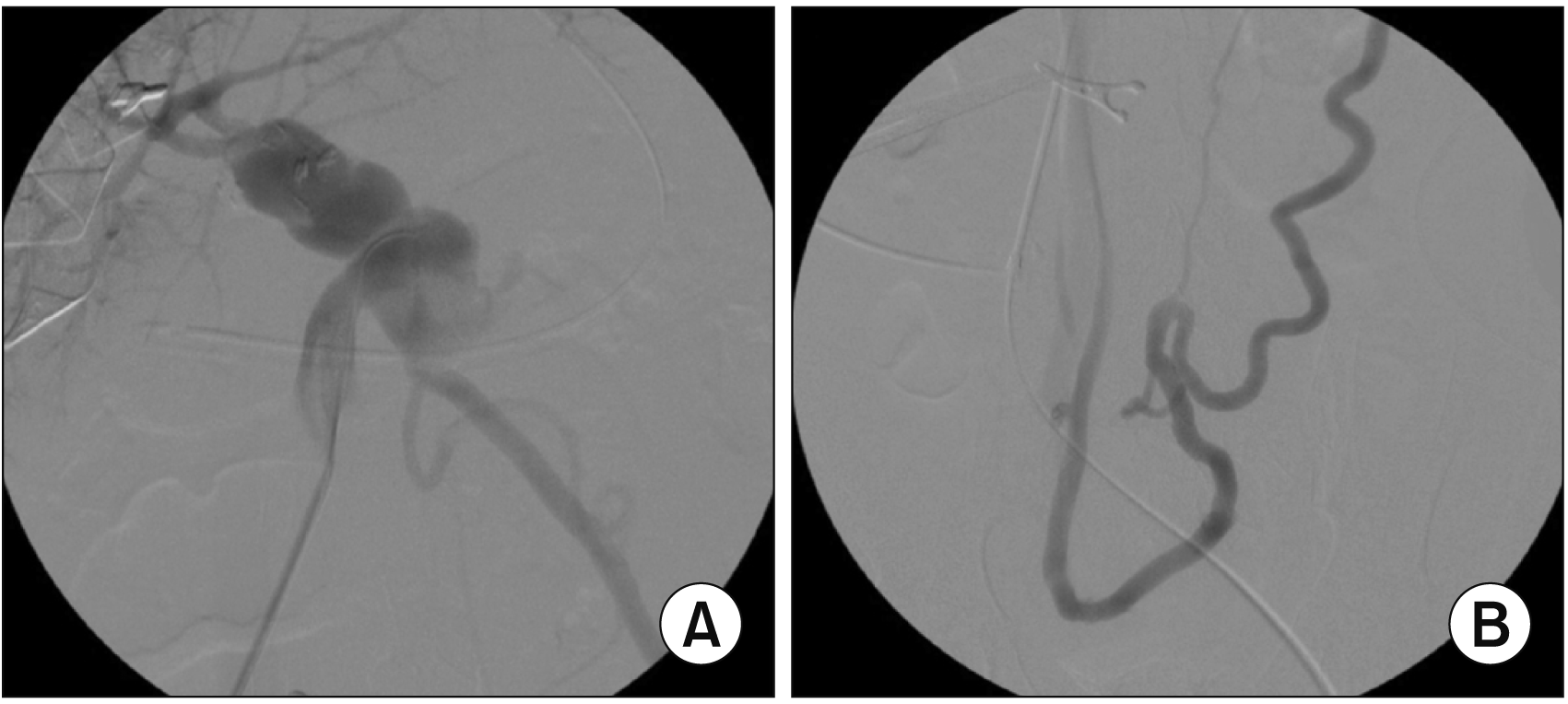
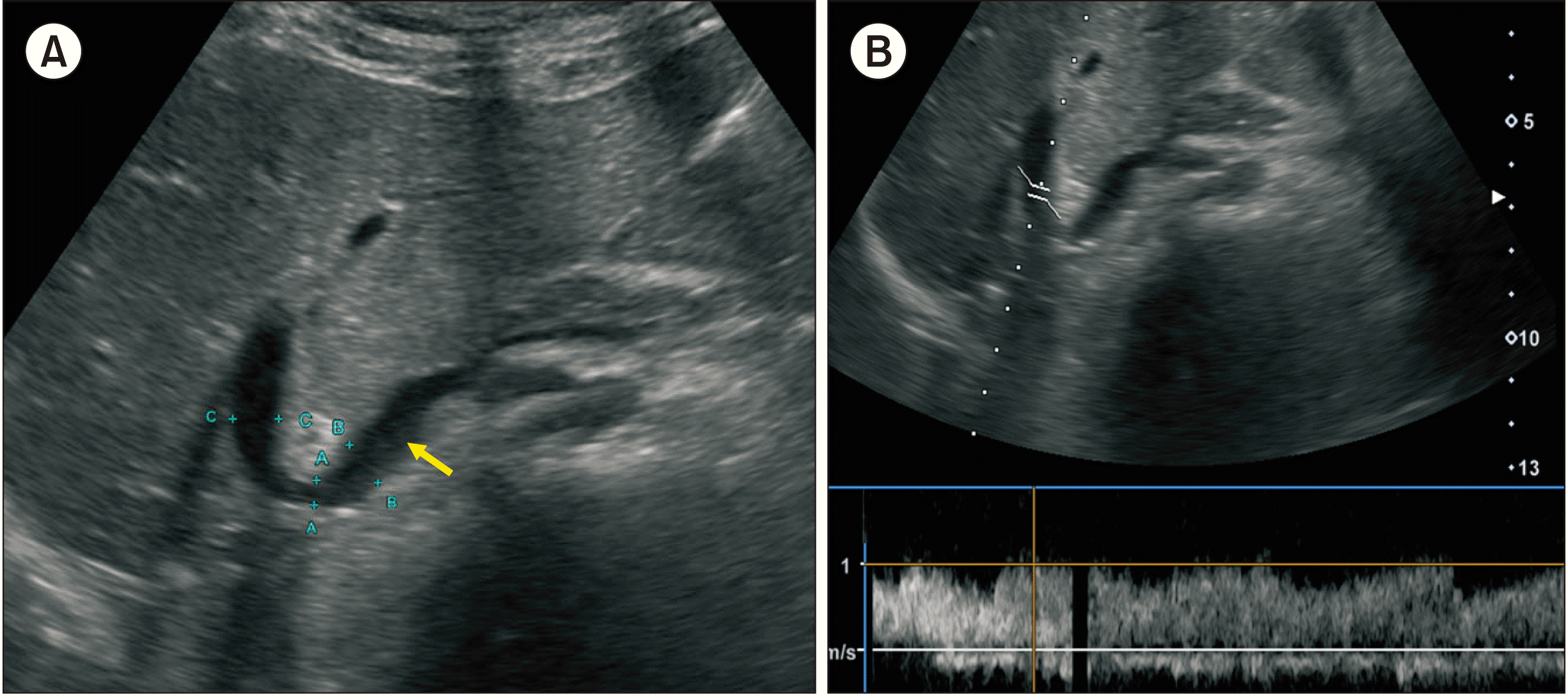 | Fig. 9Posttransplant Doppler ultrasonography at 21 days after transplantation. (A) The contour of portal vein anastomosis appears smooth and streamlined with slight stenosis at the anastomosis site. Arrow indicates the interposed iliac vein conduit. (B) The portal vein blood flow is well maintained. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download