INTRODUCTION
Primary hepatic liposarcoma is extremely rare, accounting for only 0.1% to 2% of primary malignant liver tumors [
1]. Liposarcoma originating from the mesenchymal tissue is typically found in the shoulder, extremities, and the retroperitoneum space [
2]. Primary hepatic liposarcoma can be divided into five subtypes (myxoid, well-differentiated, dedifferentiated, pleomorphic, and myxoid pleomorphic), with pleomorphic liposarcoma being the rarest subtype [
3]. The clinical presentation of primary hepatic liposarcoma is highly variable. Early diagnosis of liver liposarcoma is challenging because most patients remain asymptomatic until mass effects such as abdominal pain, abdominal distention, or obstructive jaundice symptoms emerge. Some might experience fever, nausea, vomiting, and weight loss. Physical examination might reveal a palpable abdominal mass. A computed tomography (CT) scan may provide certain typical characteristics of liver liposarcoma, making it the best tool to evaluate the resectability of liver sarcoma. Complete resection with clear margin is likely a curative therapy. To the best of our knowledge, only two cases of primary pleomorphic liver liposarcoma have been reported in the literature. Here, we present two more cases of primary pleomorphic liver liposarcoma with successful curative resection at our center. Written informed consent was obtained from both patients for publication of this case report and accompanying images.
Go to :

DISCUSSION
Liposarcoma is one of common soft tissue malignancies that occur in the extremities and visceral areas, particularly in the retroperitoneum. Metastatic spread of soft tissue liposarcomas is relatively common, with liver involvement in 10% of cases [
2]. However, primary liver liposarcoma is extremely rare, accounting for only 0.1% to 2% of primary malignant liver tumors. Classification of liposarcoma is based on a combination of two basic histological aspects of the tumor: (1) the stage of lipoblast differentiation based on relative amounts of lipids in the cells and myxoid materials in extracellular spaces; and (2) the overall degree of cellularity and cellular pleomorphism. There are five recognized subtypes: myxoid, well differentiated, dedifferentiated, pleomorphic, and myxoid pleomorphic [
3]. To date, only 15 cases of primary liver liposarcoma have been reported in the literature (
Table 1). Hence, our knowledge towards this disease remains scarce. Apparently, its long-term prognosis and survival are poor. Among reported cases (
Table 1), there was no sex (9 females: 7 males) predilection. It appearred to be more common at the left lobe of the liver. The age range was between 2 and 86 years old, with a median age of 54 years old. The myxoid subtype appeared to be the commonest.
Table 1
Characteristics of previously reported cases of primary liposarcoma of the liver
|
No |
Case source |
Age (yr) /sex |
Location |
WHO classification |
Presentation |
Management |
Outcome |
|
1 |
Kuo et al. [4] |
61/F |
Right lobe |
Myxoid |
Fever, nausea, vomiting, jaundice, tea coloured urine and loss of weight |
Right hemihepatectomy |
5 mon survival with recurrence |
|
2 |
Naik et al. [5] |
42/M |
Left lobe |
Pleomorphic |
Abdominal pain, loss of weight and palpable mass |
Extended left lateral hepatectomy |
- |
|
3 |
Wolloch et al. [8] |
22/F |
Right lobe |
- |
- |
Right lobectomy |
Survival 46 days |
|
4 |
Kim and Reyes [9] |
86/M |
Right lobe |
Myxoid |
Right sided abdominal pain, dyspnea, cough and right shoulder pain for 1 yr |
Supportive treatment |
Died rapidly |
|
5 |
Kim et al. [10] |
30/F |
Left lobe |
Dedifferentiated |
Abdominal pain and dyspnea for 4 mon |
Left lateral subsegmentectomy |
10 mon disease free |
|
6 |
Soares et al. [11] |
2/M |
Hilum |
- |
Fever, jaundice, choluria, abdominal mass and anorexia for 1 mon |
Laparotomy biopsy, and chemotherapy |
5 mon survival |
|
7 |
Wright et al. [12] |
3/M |
Hilum |
Myxoid |
Right upper abdominal mass |
Wide local excision |
Disease free 12 yr. Recurrence. |
|
8 |
Aribal and Berberoglu [13] |
48/F |
Hilum |
Myxoid |
Right abdominal pain |
Laparotomy biopsy, and chemotherapy |
- |
|
9 |
Nelson et al. [14] |
54/F |
Right and left lobe |
Myxoid |
Abdominal distension, pain, weight loss, nausea and vomiting for 1 yr |
Laparotomy and biopsy |
Post surgery bleeding and death |
|
10 |
Kim et al. [15] |
63/F |
Left lobe |
Well differentiated |
Abnormal serum liver enzyme |
Left hemihepatectomy |
- |
|
11 |
Nakhai and Motabar [16] |
21/F |
Right lobe |
- |
Fever, dyspnea and abdominal pain for 3 mon |
Right hemihepatectomy and chemotherapy |
9 mon survival |
|
12 |
Lin et al. [17] |
64/M |
Left lobe |
Myxoid |
Leg pain for 1 mon |
Left hemihepatectomy |
4 yr disease free |
|
13 |
Binesh et al. [18] |
83/F |
Left lobe |
Myxoid |
Nausea, vomiting, palpable painful epigastric mass, loss of weight for 6 mon |
Tumour resection and chemotherapy |
19 mon disease free. Recurrence |
|
14 |
Chen et al. [19] |
43/M |
Left lobe |
Pleomorphic |
Abdominal distention, pain, nausea, vomiting, weight loss for 1 yr |
Left hemihepatectomy |
9 mon disease free. Recurrence |
|
15 |
Rayya [20] |
59/M |
Right lobe |
Myxoid |
Nonspecific right hypochondrium pain, weight loss for 9 mon |
Lateral partial right hepatectomy |
More than 3 mon survival |
|
16 |
Our 1st case |
57/F |
Left lobe |
Pleomorphic |
Abdominal discomfort and distention for 2 wk |
Wide local excision |
More than 6 mon survival (remains disease free) |
|
17 |
Our 2nd case |
65/M |
Left lobe |
Pleomorphic |
Abdominal mass, weight loss for 2 mon |
Wide local excision |
More than 3 mon survival |

Early diagnosis of primary liver liposarcoma is difficult because a tumor in the liver is usually undetected unless it is large. In addition, its symptoms are non-specific (
Table 1). Most symptoms are due to the mass effect of the tumor on nerves, vessels, the biliary tract, and intestines [
4]. Common symptoms reported are fever, nausea, vomiting, loss of weight, jaundice, abdominal distention, abdominal pain, and palpable abdominal mass. In our case series, both patients presented with abdominal distention and a palpable abdominal mass. However, there was no obstructive jaundice symptom because the tumor was confined to the liver lobe without having any compressive effect on the biliary duct.
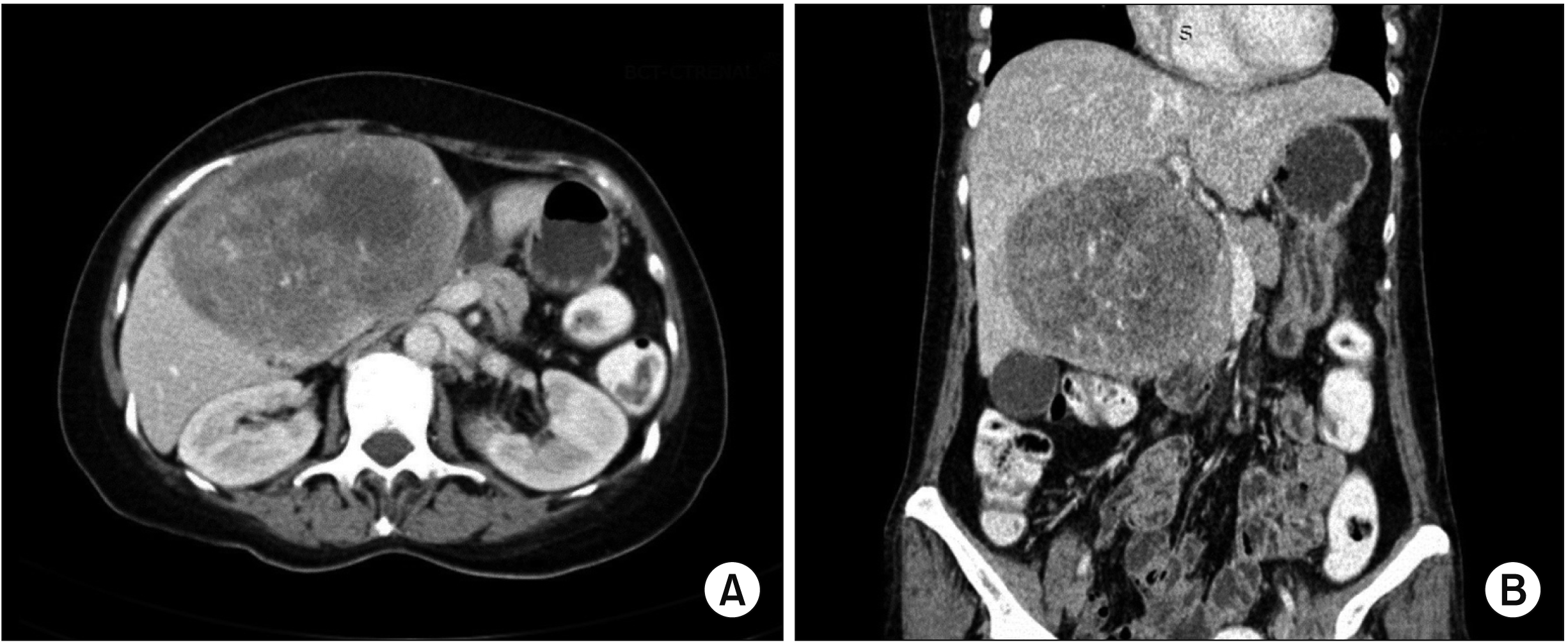
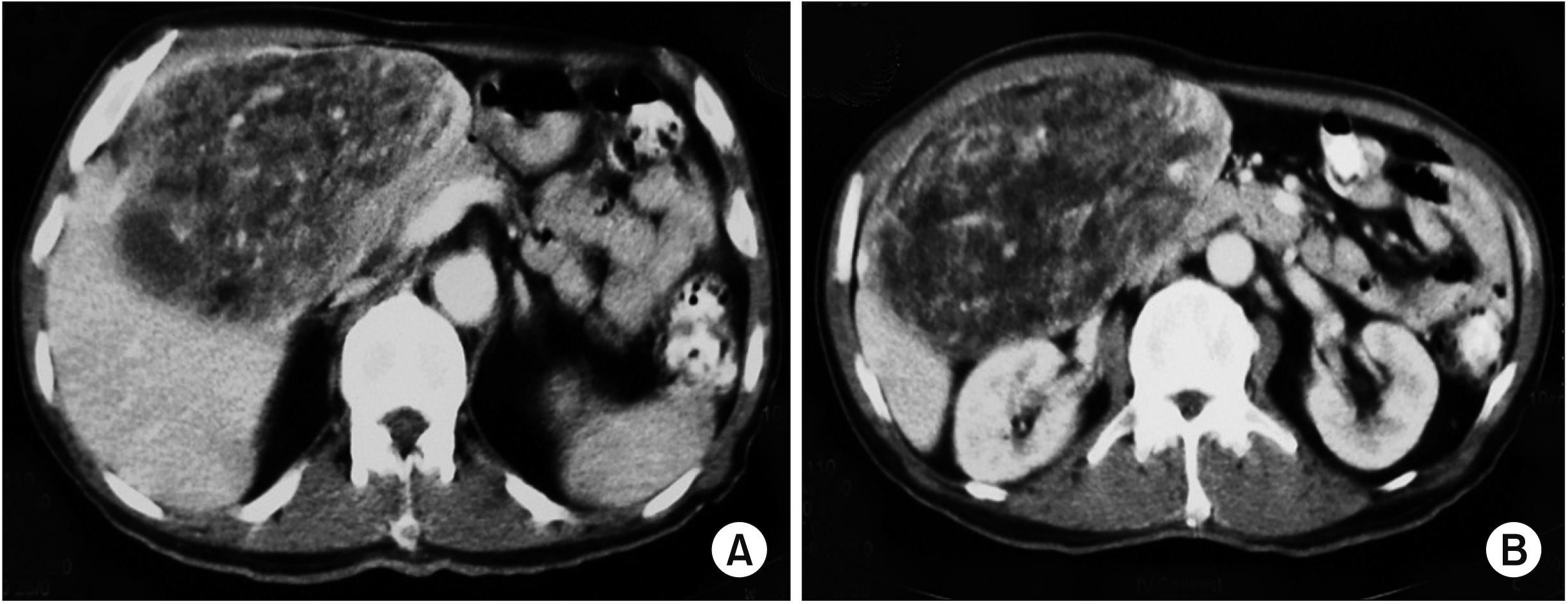
A CT scan of the abdomen is the imaging modality of choice for identifying certain typical characteristics of liposarcoma [
4-
6] and for evaluating the resectablitiy of liver liposarcoma prior to surgery. It remains a challenge to differentiate liposarcoma from other liver diseases due to their varied histological compositions including areas of necrosis and hemorrhage.
For well-differentiated liposarcoma, it has a predominantly adipose mass containing non-lipomatous components. The fat component is usually > 75%, while non-lipomatous components are most often seen as prominent thick septa (> 2 mm) with or without nodularity. There might be a presence of focal nodular or globular non-adipose area [
6]. For dedifferentiating liposarcoma, it has features similar to those of well-differentiated liposarcoma. However, it has a focal, nodular non-lipomatous region over 1 cm in size. Myxoid liposarcoma may appear normal or as a non-specific soft tissue mass. It has less calcification and radiolucent fat. The fat component is usually < 10% of the lesion. It is often seen in the septa (lacy or linear pattern) or as subtle small nodules in the lesion [
6].
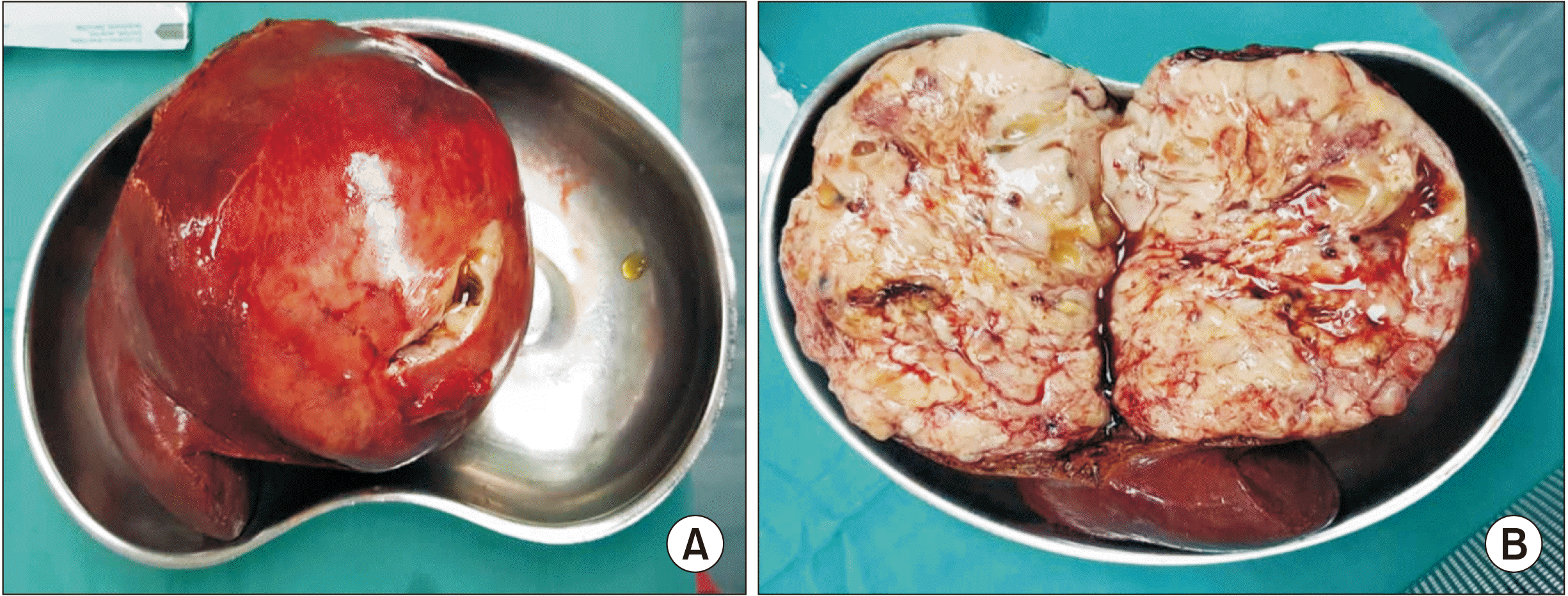
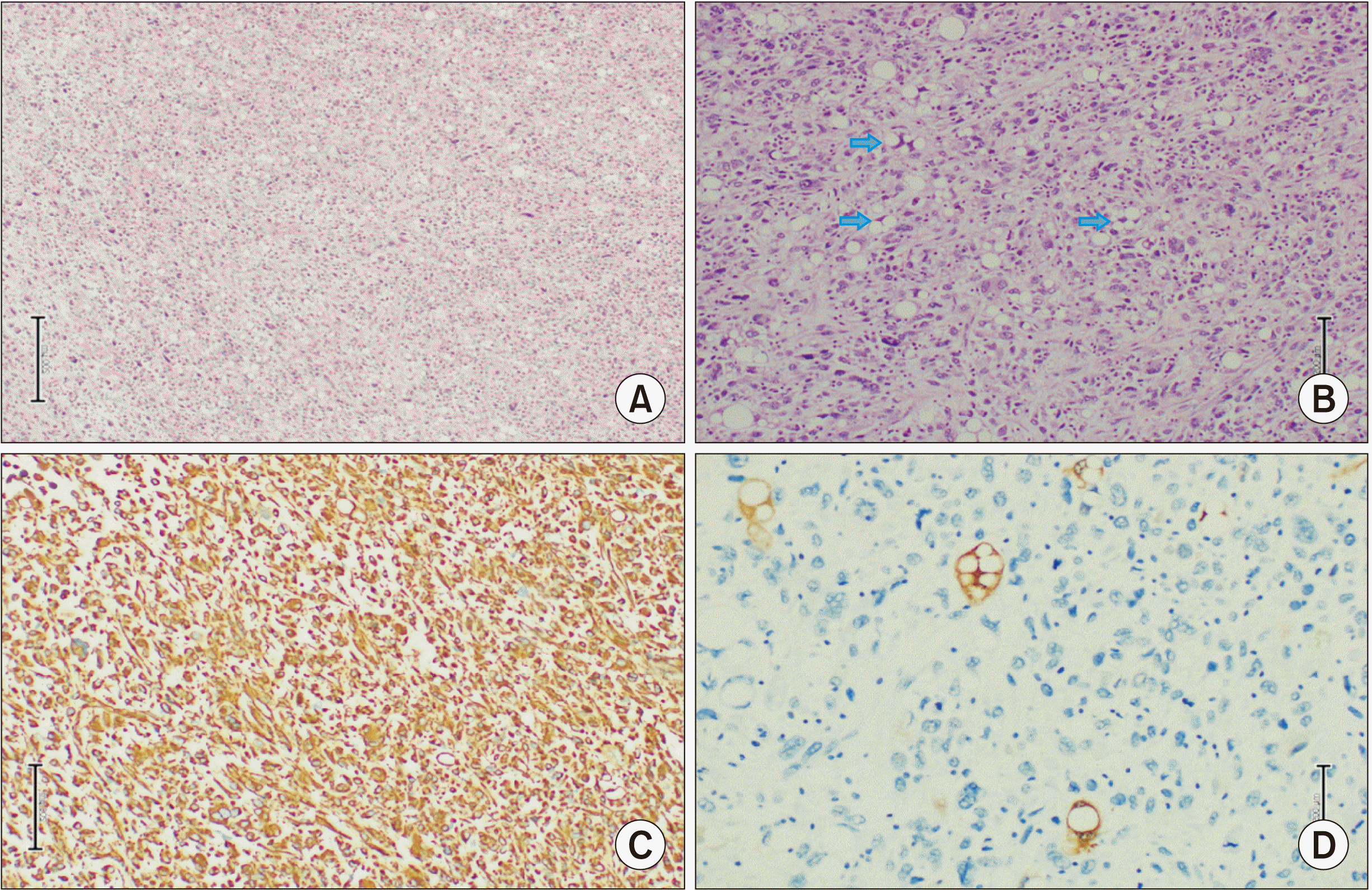
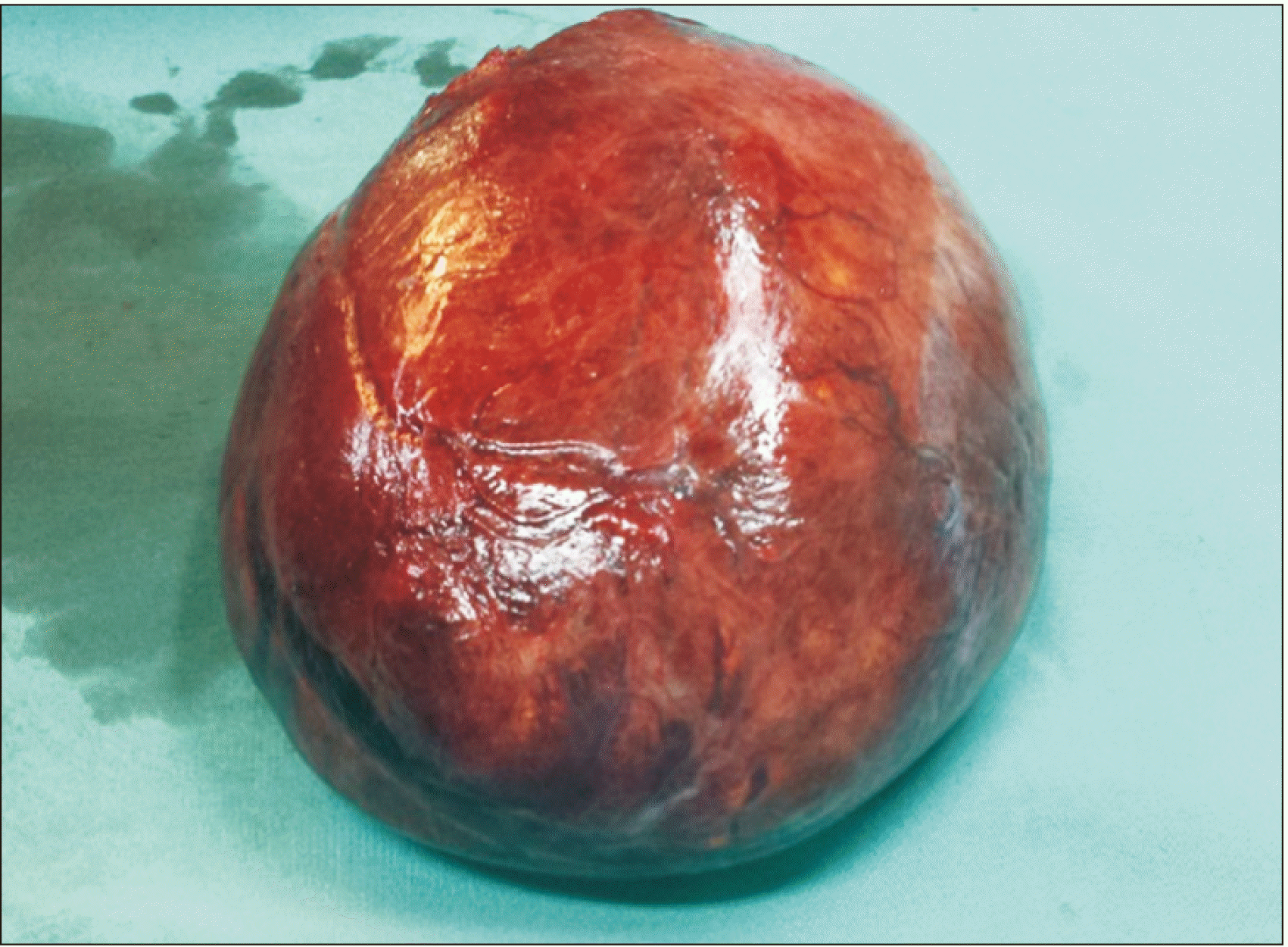
Pleomorphic liposarcoma is typically presented as a non-specific soft tissue mass. It is usually associated with an area of necrosis and hemorrhage, accounting for the prominent heterogenecity seen in these lesions (similar to the one in our 1st case). Furthermore, it seldom contains adipose tissues, reflecting a higher degree of anaplasia of the lesion [
6]. Both our cases involved pleomorphic type of hepatic liposarcoma, although their CT features were entirely different. The 1st case demonstrated a predominantly solid nodule mass with an area of necrosis and hemorrhage, while the 2nd case showed a mixed soft tissue and fat mass. These non-specific features of pleomorphic liposarcoma can make imaging diagnosis difficult. Tumor markers (such as AFP, CEA, or cancer antigen 19-9) were not increased. Liposarcoma should be one of our differential diagnoses when dealing with a liver mass.
Based on the literature review, the mainstay of treatment is curative surgical resection with clear margins [
7]. However, the disease is accompanied by a high rate of tumor recurrence with poor prognosis [
4,
5,
8-
20]. Palliative treatment should be offered for those with advanced liposarcoma and metastases. The role of chemotherapy and radiotherapy in hepatic liposarcoma remains less defined with a lack of strong evidence. Among the 15 cases reported, only four of them [
11,
13,
16,
18] underwent chemotherapy post-surgical resection, with survival duration ranging from 5 to 19 months. Our patient in the 1st case is disease free currently at six months post-surgical resection of the hepatic pleomorphic liposarcoma. She is still on a regular follow up schedule. However, the patient in the 2nd case had lost his follow up at three months post-surgery.
Table 1 shows outcomes of all reported primary hepatic liposarcoma cases in the literature.
In conclusion, our two cases highlight our experience on the clinical course of primary hepatic liposarcoma which is extremely rare. Although there is no clear consensus, surgical resection with clear margin remains to be the mainstay of treatment. Radiotherapy and chemotherapy in hepatic liposarcoma remain uncertain. They might play a role in potentially achieving a long-term survival. Additional strong evidence is required. Based on cases reported in the literature, prognosis of this disease is poor, showing a high recurrence rate.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download