Abstract
Background
Methods
Results
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS
Yoon S collected the samples, analyzed and interpreted the data, and wrote the draft; Moon HW conceived the study and finalized the draft; Kim H, Hur M, and Yun YM discussed the data and reviewed the manuscript. All authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
REFERENCES
Fig. 1
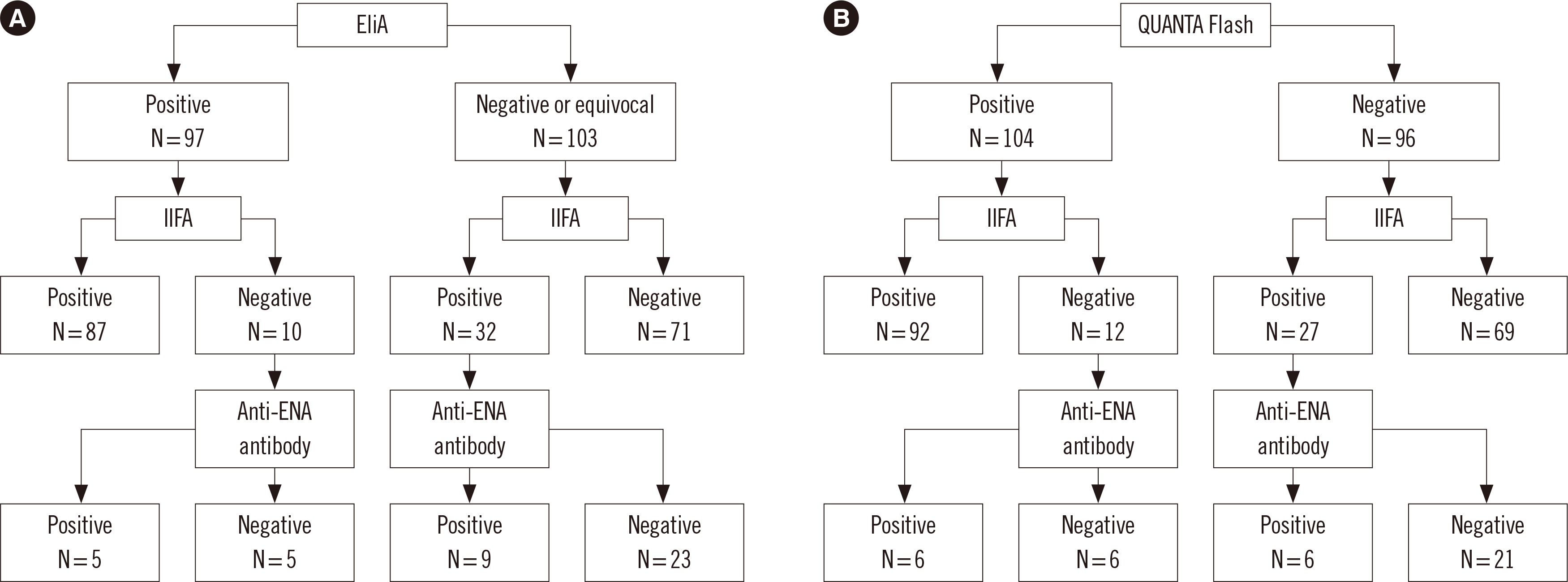
Table 1
Abbreviations: AARD, antinuclear antibody-associated rheumatic disease; CTD, connective tissue disease; CU, chemiluminescent units; IIFA, indirect immunofluorescence assay; MCTD, mixed connective tissue disease; N, number of samples; PM/DM, polymyositis/dermatomyositis; RA, rheumatoid arthritis; SjS, primary Sjögren’s syndrome; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
Table 2
| Case number | Diagnosis | EliA* | QUANTA Flash† | IIFA‡ | Anti-ENA antibodies |
|---|---|---|---|---|---|
| 1 | SLE | (P) 7.5 | (P) > 200.0 | (N) 1 : 40 | SS-A (Ro60) |
| 2 | SLE | (P) 7.3 | (P) 125.5 | (N) 1 : 40 | SS-A (Ro60) |
| 3 | CTD | (N) 0.4 | (P) > 200.0 | (N) Weak | SS-A (Ro60) |
| 4 | SjS | (P) 1.5 | (P) 29.5 | (N) 1 : 40 | SS-A (Ro60) |
| 5 | SLE | (N) 0.4 | (N) 8.1 | (P) 1 : 640 | Nucleosome |
| 6 | SLE | (N) 0.5 | (N) 9.8 | (P) 1 : 320 | Nucleosome |
| 7 | SLE | (P) 8.0 | (P) 49.1 | (N) Weak | Histone, SS-A (Ro60) |
| 8 | SLE | (P) 5.7 | (P) 111.4 | (N) 1 : 40 | Ribosomal-P, SS-A (Ro60) |
| 9 | SLE | (P) 4.7 | (N) 17.7 | (P) 1 : 160 | dsDNA, nRNP, ribosomal-P, Sm |
| 10 | SLE | (N) 0.9 | (N) 8.7 | (P) 1 : 320 | Histone |
| 11 | SLE | (N) 0.7 | (N) 11.0 | (P) 1 : 80 | Scl-70 |
| 12 | SLE | (N) 0.6 | (N) 11.7 | (P) 1 : 1,280 | ND |
| 13 | SLE | (N) 0.4 | (N) 10.8 | (P) 1 : 320 | ND |
| 14 | SLE | (N) 0.3 | (N) 15.4 | (P) 1 : 80 | ND |
| 15 | SLE | (N) 0.1 | (N) 10.3 | (P) 1 : 80 | ND |
| 16 | SLE, SjS | (N) 0.1 | (N) 8.8 | (P) 1 : 80 | ND |
| 17 | CTD | (N) 0.4 | (N) 2.6 | (P) 1 : 320 | ND |
| 18 | CTD | (N) 0.2 | (N) 5.7 | (P) 1 : 80 | ND |
| 19 | Unclassified CTD | (N) 0.2 | (N) 8.4 | (P) 1 : 160 | ND |
| 20 | Collagen disease | (N) 0.1 | (N) 3.9 | (P) 1 : 320 | ND |
| 21 | RA | (N) 0.1 | (N) 8.1 | (P) > 1 : 1,280 | ND |
| 22 | RA | (N) 0.1 | (N) 7.4 | (P) 1 : 160 | ND |
| 23 | SjS | (N) 0.4 | (N) 4.1 | (P) 1 : 80 | ND |
| 24 | SSc | (N) 0.3 | (N) 5.5 | (P) 1 : 320 | ND |
| 25 | Arthralgia | (N) 0.3 | (N) 6.6 | (P) 1 : 80 | ND |
| 26 | Arthrosis | (N) 0.2 | (N) 3.8 | (P) 1 : 80 | ND |
| 27 | Cough | (N) 0.1 | (N) 4.5 | (P) 1 : 160 | ND |
| 28 | Dry mouth | (N) 0.2 | (N) 7.5 | (P) 1 : 160 | ND |
| 29 | Hemoptysis | (N) 0.2 | (N) 6.9 | (P) 1 : 80 | ND |
| 30 | ILD | (N) 0.2 | (N) 3.8 | (P) 1 : 80 | ND |
| 31 | Oral ulcer | (N) 0.2 | (N) 3.0 | (P) 1 : 160 | ND |
| 32 | R/O FFA | (N) 0.2 | (N) 4.2 | (P) 1 : 160 | ND |
| 33 | SLE | (N) 0.2 | (P) 24.1 | (N) Weak | ND |
| 34 | SLE | (N) 0.9 | (P) 24.1 | (N) 1 : 40 | ND |
| 35 | Arthrosis | (N) 0.1 | (P) 44.7 | (N) 1 : 40 | ND |
| 36 | Rash | (N) 0.2 | (P) 131.7 | (N) Negative | ND |
| 37 | CTD | (P) 1.1 | (N) 13.6 | (N) Weak | ND |
| 38 | OA | (P) 1.7 | (N) 7.8 | (N) 1 : 40 | ND |
| 39 | SLE | (P) 1.7 | (N) 12.7 | (N) 1 : 40 | ND |
| 40 | RA | (P) 1.1 | (P) 24.6 | (N) 1 : 40 | ND |
| 41 | Thrombocytopenia | (P) 1.2 | (P) 51.2 | (N) 1 : 40 | ND |
| 42 | CTD | (P) 1.1 | (N) 6.8 | (P) 1 : 80 | ND |
*EliA: >1.0 ratio, positive; 0.7–1.0 ratio, equivocal; <0.7 ratio, negative. †QUANTA Flash: ≥20.0 CU, positive; <20.0 CU, negative. ‡IIFA: ≥1:80, positive; <1:80, negative.
Abbreviations: CTD, connective tissue disease; CU, chemiluminescent units; ENA, extractable nuclear antigen; FFA, Frontal fibrosing alopecia; IIFA, indirect immunofluorescence assay; ILD, interstitial lung disease; N, negative; ND, not detected; OA, osteoarthritis; P, positive; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SjS, Sjögren’s syndrome; SSc, systemic sclerosis; R/O, rule out; SjS, primary Sjögren’s syndrome; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download