Abstract
Background
Since 2017, automated assays have been used in most clinical laboratories for anti-Müllerian hormone (AMH) level measurement. We evaluated the analytical performance of the newly developed automated fluorescent immunoassay system (AFIAS) AMH assay (Boditech Med, Gangwon-do, Korea) in comparison with the Roche Elecsys and Beckman Coulter Access 2 AMH assays.
Methods
Analytical performance of the AFIAS AMH assay was assessed in terms of linearity, repeatability, and within-laboratory precision (CV%) using human recombinant AMH samples according to the Clinical and Laboratory Standards Institute (CLSI) guidelines EP05 and EP06. Using 293 serum samples collected from an infertility clinic, the AMH levels were compared across AFIAS, Elecsys, and Access 2 AMH assays according to the CLSI EP09 guidelines.
Results
The AFIAS AMH assay results were linear across the measurement range of 0.420–72.386 pmol/L AMH, with repeatability of 6.341%. CV% of the AFIAS AMH assay for three levels of control, 1.786, 7.143, and 56.857 pmol/L, were 5.801%, 5.714%, and 6.228%, respectively. The results of the three AMH assays showed strong correlation AFIAS and Elecsys [slope, 1.055 (95% confidence interval (CI), 1.022–1.088) and Spearman’s rho, 0.978 (95% CI, 0.973–0.983)], Elecsys and Access 2 [slope, 0.813 (95% CI, 0.791–0.834) and Spearman’s rho, 0.986 (95% CI, 0.983–0.989)], and AFIAS and Access 2 [slope, 0.836 (95% CI, 0.821–0.853) and Spearman’s rho, 0.984 (95% CI, 0.980–0.988)].
Anti-Müllerian hormone (AMH) is a homodimeric glycoprotein linked by disulfide bonds with a molecular weight of 140 kDa and belongs to the transforming growth factor-β (TGF-β) superfamily. AMH production by embryonic Sertoli cells induces Müllerian ducts; thus, this hormone plays a vital role in male sex differentiation [1]. In women, granulosa cells of the small antral and pre-antral follicles produce AMH, and the AMH expression pattern is suggested to correlate with the number of early growing follicles [2]. Therefore, AMH in women may serve as an indicator of the ovarian reserve and a predictor of the ovarian response to hyperstimulation [2-8].
Since the development and market introduction of ELISAs by Diagnostic Systems Lab (DSL, Webster, TX, USA) and Immunotech (IOT, Marseilles, France) two decades ago, several AMH immunoassays, including automated assays, have been developed [9-12]. The Elecsys AMH assay (Roche Diagnostics, Basel, Switzerland) and the Access 2 AMH assay (Beckman Coulter, Brea, CA, USA) are widely used automated assays. Owing to their large assay capacity, they are suitable for clinical laboratories [12]. However, small in vitro fertilization (IVF) laboratories and clinics require automated assays combined with point-of-care (POC) instruments for performing small numbers of assays.
Recently, Boditech Med (Chuncheon, Korea) developed an automated fluorescent immunoassay system (AFIAS) AMH assay, which is an automated one-step immunoassay with time-resolved fluorescence detection using an all-in-one cartridge consisting of all factory-calibrated reagents. The AFIAS AMH assay uses only 100 μL of blood and takes only 3–15 minutes to deliver the result. We evaluated the analytical performance of the AFIAS AMH assay in comparison with that of the Elecsys and Access 2 AMH assays.
Serum samples were collected prospectively at the infertility clinic of MizMedi Hospital, Seoul, Korea, between December 6, 2018 and July 20, 2019, with approval of the Ethics Committee on Human Subjects of MizMedi Hospital (MMIRB2018-10). In total, 332 participants (age range, 19–49 years) were enrolled in this study and provided informed consent. Thirty-nine participants were excluded from the analyses for the following reasons: nine participants withdrew consent, four were not Korean, and 26 had serum AMH levels below or above the measurement range of the AFIAS AMH assay. Collected serum samples from the remaining 293 women (mean age, 34.27±5.13 years) were aliquoted into two tubes for standard laboratory AMH level measurement. For assay comparison, samples were stored in the freezer (–20°C) until AMH level measurement. Sample stability was assessed by analyzing the differences in AMH levels between fresh serum samples (day 0) and frozen/thawed samples using a reference instrument (Beckman Coulter Access 2), according to the European Medicines Agency (EMA) guidelines [13].
We followed the CLSI EP06 guidelines to measure the linearity of the AFIAS AMH assay [14]. We prepared a series of human recombinant AMH samples with known AMH levels (Boditech Med), ranging from a low (near the lower limit of quantification) to a high level (close to the upper limit of quantification). We analyzed each sample in 10 replicates and generated linear and non-linear regression equations [14].
Repeatability and within-laboratory precision of the AFIAS AMH assay were calculated using three control human recombinant AMH samples with known AMH levels according to a modified protocol presented in the CLSI EP05 guidelines: 20 days, two runs daily, and six replicates per run [15].
The results obtained using the AFIAS, Elecsys, and Access 2 AMH assays were compared using Passing–Bablok regression analysis and Bland–Altman methods, according to the CLSI EP09 guidelines [16]. Statistical analyses for Passing–Bablok regression, Bland–Altman plot, and Spearman’s correlation were conducted using MedCalc software (version 19.2.0, MedCalc Software bvba, Ostend, Belgium) and R software (R Core Team 2014, Vienna, Austria). A P value <0.05 was considered statistically significant.
Linear results were obtained across the measurement range of 0.420–72.386 pmol/L, with repeatability of 6.341% for all samples (Table 1). The repeatability and within-laboratory precision of the AFIAS AMH assay were 5.607%–5.659% and 5.714%–6.228%, respectively, which were within the ranges reported by the manufacturer (Table 2).
Age-specific AMH levels in the 10th, 50th, and 90th percentiles, with 95% confidence intervals (CIs) calculated from the measurements obtained with each assay are shown in Table 3. The AMH levels obtained using the AFIAS and Elecsys AMH assays were similar (P=0.807), whereas the Access 2 AMH assay yielded higher values (P=0.009) (Fig. 1).
We redetermined the AMH level in each sample using the Access 2 AMH assay to ensure sample stability under storage at –20°C. The measured levels for the thawed samples tended to be slightly higher (measured level after thawing=1.066×measured level for fresh sample - 0.017, Spearman’s correlation coefficient r=0.996), but the differences were acceptable (96% of the samples showed a difference under 20%, while 76% samples showed a difference under 10%) (Fig. 2).
Strong correlations with no significant differences were found among the results of the three AMH assays (Fig. 3): The slope and Spearman’s rho were 1.055 (95% CI, 1.022–1.088) and 0.978 (95% CI, 0.973–0.983) between AFIAS and Elecsys, 0.813 (95% CI, 0.791–0.834) and 0.986 (95% CI, 0.983–0.989) between Elecsys and Access 2, and 0.836 (95% CI, 0.821–0.853) and 0.984 (95% CI, 0.980–0.988) between AFIAS and Access 2, respectively. The Bland–Altman plot showed no significant systemic error across the AMH level measurement range among assays.
We found competent repeatability, within-laboratory precision, and adequate linearity of the recently developed AFIAS AMH assay with comparable measurement values to those of the Elecsys and Access 2 AMH automated assays.
AMH level measurement is a popular approach to assess the ovarian reserve and identify extreme responses before ovarian stimulation in IVF treatment, because the AMH level highly correlates with the antral follicle count [17, 18]. The usual starting point of ovarian stimulation for IVF is menstrual cycle day (MCD) 2 or 3. The starting dose of gonadotropin in ovarian stimulation is adjusted according to the ovarian reserve, and hormonal levels are checked in the early follicular phase (MCD 2–3). Determination of the levels of AMH and other hormones on the starting day of ovarian stimulation would greatly assist timely adjustment of gonadotropin dose. The impact of the proverbial biological clock, the so-called “ovarian age,” on egg quality and quantity is also a concern in relatively older (>40 years) patients in IVF clinics, who do not want to delay ovarian stimulation. Hence, reducing the laboratory turn-around time of assays for AMH and other hormones is preferable.
In small- to medium-sized IVF clinics, blood samples are usually sent out to reference laboratories. Outsourcing of hormonal assays not only takes more time but may also affect the quality of the laboratory results. AMH level measurement with a short turn-around time would significantly contribute to patient satisfaction. To our knowledge, the AFIAS AMH assay is the only POC automated AMH assay currently available, offering an affordable on-site laboratory assay for small- to medium-sized IVF clinics with reduced laboratory assay capacity.
This study is the first to compare three automated AMH assays (AFIAS, Elecsys, and Access 2) using serum samples from patients. As the AFIAS AMH assay results showed strong correlations with the results of the other two assays (Fig. 3), it could serve as a convenient alternative. The distributions of AMH levels measured using the AFIAS and Elecsys AMH assays were similar, whereas the Access 2 AMH assay showed a slightly wider distribution (Fig. 1). Similar distribution patterns among assays were found for age-specific AMH levels (Table 3). This indicates that assay-specific AMH levels need to be carefully interpreted. Since AMH level measurement has not yet been standardized [17, 18], it is preferable to use the same assay when testing AMH level serially for the same patient.
We only considered age and AMH level for each participant and did not analyze correlations of AMH levels with clinical profiles. Therefore, we cannot offer any practical suggestion regarding the most suitable AMH level for infertility treatment. Further, serum samples were obtained only from participants who had registered for infertility treatment. Therefore, our data do not indicate age-specific AMH levels in the general population of Korean women.
Despite these limitations, we showed the competent repeatability and within-laboratory precision and adequate linearity of AFIAS AMH assay, and its performance was satisfactory compared with the widely used Elecsys Access 2 AMH assays. Therefore, the AFIAS AMH assay can be an alternative to existing immunoassays.
Notes
AUTHOR CONTRIBUTIONS
Han A designed the study, collected data, and wrote and revised the manuscript. Suh B supervised the laboratory work and proofread the manuscript. Lee G designed the study and collected the samples. Lee Y and Kim S collected samples. All authors read and approved the final manuscript.
REFERENCES
1. Carmina E, Stanczyk F, et al. Strauss JF, Barbieri RL, editors. 2018. Evaluation of hormonal status. Yen and Jaffés reproductive endocrinology. 8th ed. Elsevier;Amsterdam: p. 900–1.

2. Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. 2012; Serum anti-Müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 97:4650–5. DOI: 10.1210/jc.2012-1440. PMID: 22993032. PMCID: PMC3683801.

3. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. 2002; Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 77:357–62. DOI: 10.1016/S0015-0282(01)02993-4. PMID: 11821097.

4. van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. 2002; Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 17:3065–71. DOI: 10.1093/humrep/17.12.3065. PMID: 12456604.
5. Lie Fong S, Baart EB, Martini E, Schipper I, Visser JA, Themmen AP, et al. 2008; Anti-Müllerian hormone: a marker for oocyte quantity, oocyte quality and embryo quality? Reprod Biomed Online. 16:664–70. DOI: 10.1016/s1472-6483(10)60480-4. PMID: 18492370.
6. Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. 2003; Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 18:323–7. DOI: 10.1093/humrep/deg042.

7. van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, et al. 2005; Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 83:979–87. DOI: 10.1016/j.fertnstert.2004.11.029. PMID: 15820810.
8. Broer SL, Dólleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. 2011; AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. 17:46–54. DOI: 10.1093/humupd/dmq034. PMID: 20667894.

9. Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. 2010; Development of a second generation anti-Müllerian hormone (AMH) ELISA. J Immunol Methods. 362:51–9. DOI: 10.1016/j.jim.2010.08.011. PMID: 20801125.

10. Gassner D, Jung R. 2014; First fully automated immunoassay for anti-Müllerian hormone. Clin Chem Lab Med. 52:1143–52. DOI: 10.1515/cclm-2014-0022. PMID: 24622790.

11. Pearson K, Long M, Prasad J, Wu YY, Bonifacio M. 2016; Assessment of the access AMH assay as an automated, high-performance replacement for the AMH generation II manual ELISA. Reprod Biol Endocrinol. 14:8. DOI: 10.1186/s12958-016-0143-3. PMID: 26879773. PMCID: PMC4754992.

12. Pastuszek E, Lukaszuk A, Kunicki M, Mockun J, Kloss G, Malinowska I, et al. 2017; New AMH assay allows rapid point of care measurements of ovarian reserve. Gynecol Endocrinol. 33:638–43. DOI: 10.1080/09513590.2017.1306735. PMID: 28457181.

13. European Medicines Agency. Guideline on bioanalytical method validation. EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2**. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf. Updated on 21July 2011.
14. CLSI. 2020. Evaluation of the linearity of quantitative measurement procedures: A statistical approach; Approved guideline. EP06. 2nd ed. Clinical and Laboratory Standards Institute;Wayne, PA:
15. CLSI. 2014. Evaluation of precision of quantitative measurement procedures; Approved guideline. EP05. 3rd ed. Clinical and Laboratory Standards Institute;Wayne, PA:
16. CLSI. 2018. Measurement procedure comparison and bias estimation using patient samples; Approved guideline. EP09. 3rd ed. Clinical and Laboratory Standards Institute;Wayne, PA:
17. Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. 2014; The physiology and clinical utility of anti-Müllerian hormone in women. Hum Reprod Update. 20:370–85. DOI: 10.1093/humupd/dmt062.

18. Nelson SM, Pastuszek E, Kloss G, Malinowska I, Liss J, Lukaszuk A, et al. 2015; Two new automated, compared with two enzyme-linked immunosorbent, antimüllerian hormone assays. Fertil Steril. 104:1016–21.e6. DOI: 10.1016/j.fertnstert.2015.06.024. PMID: 26183313.

Fig. 1
Distribution of AMH levels measured using the AFIAS, Elecsys, and Access 2 AMH assays. Squares and bars represent the median of measured data and 95% CI.
Abbreviations: AMH, anti-Müllerian hormone; AFIAS automated fluorescent immunoassay system; CI, confidence interval.
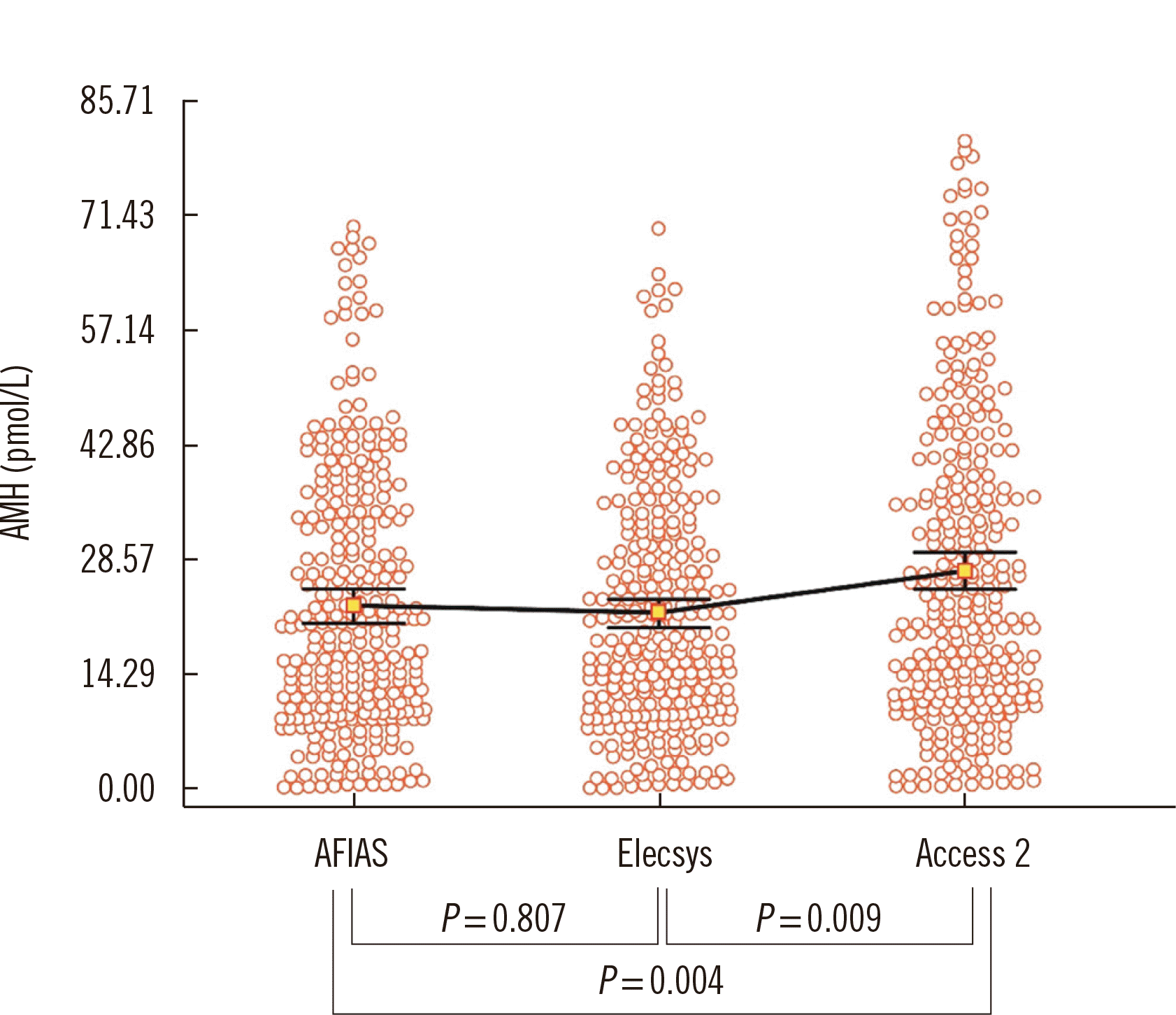
Fig. 2
Correlation between two measurements of fresh and frozen-thawed samples. (A) Scatter plots with regression line of two measurements. (B) Percent difference between two measurements. Thick and thin solid lines represent 0%, ±10%, and ±20% differences, respectively. Dotted line represents the regression line of the mean difference between the two measurements.
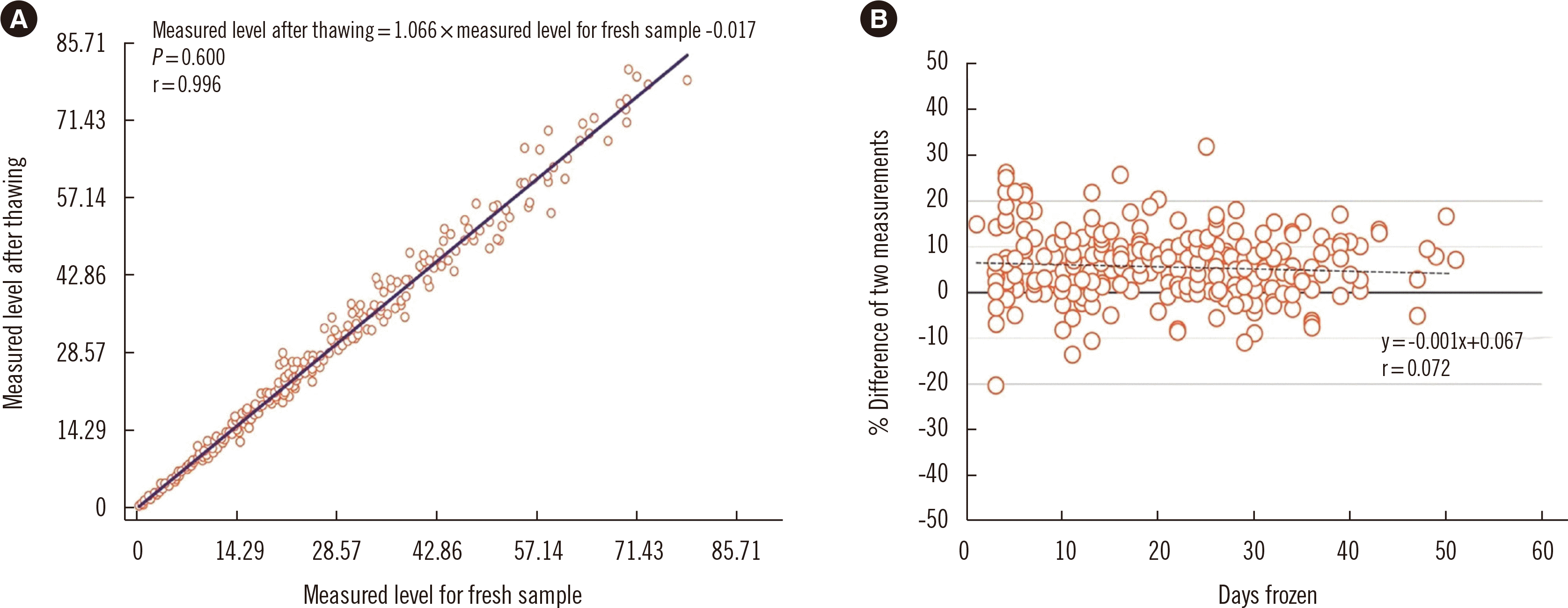
Fig. 3
Correlation analyses of the three AMH assays. (A), (B), and (C) show Passing–Bablok regression plots of the three AMH assays. Solid lines represent the regression of two measurements. (D), (E), and (F) show Bland–Altman plots of the appropriate comparison between the AMH assays.
Abbreviations: AMH, anti-Müllerian hormone; AFIAS, automated fluorescent immunoassay system.
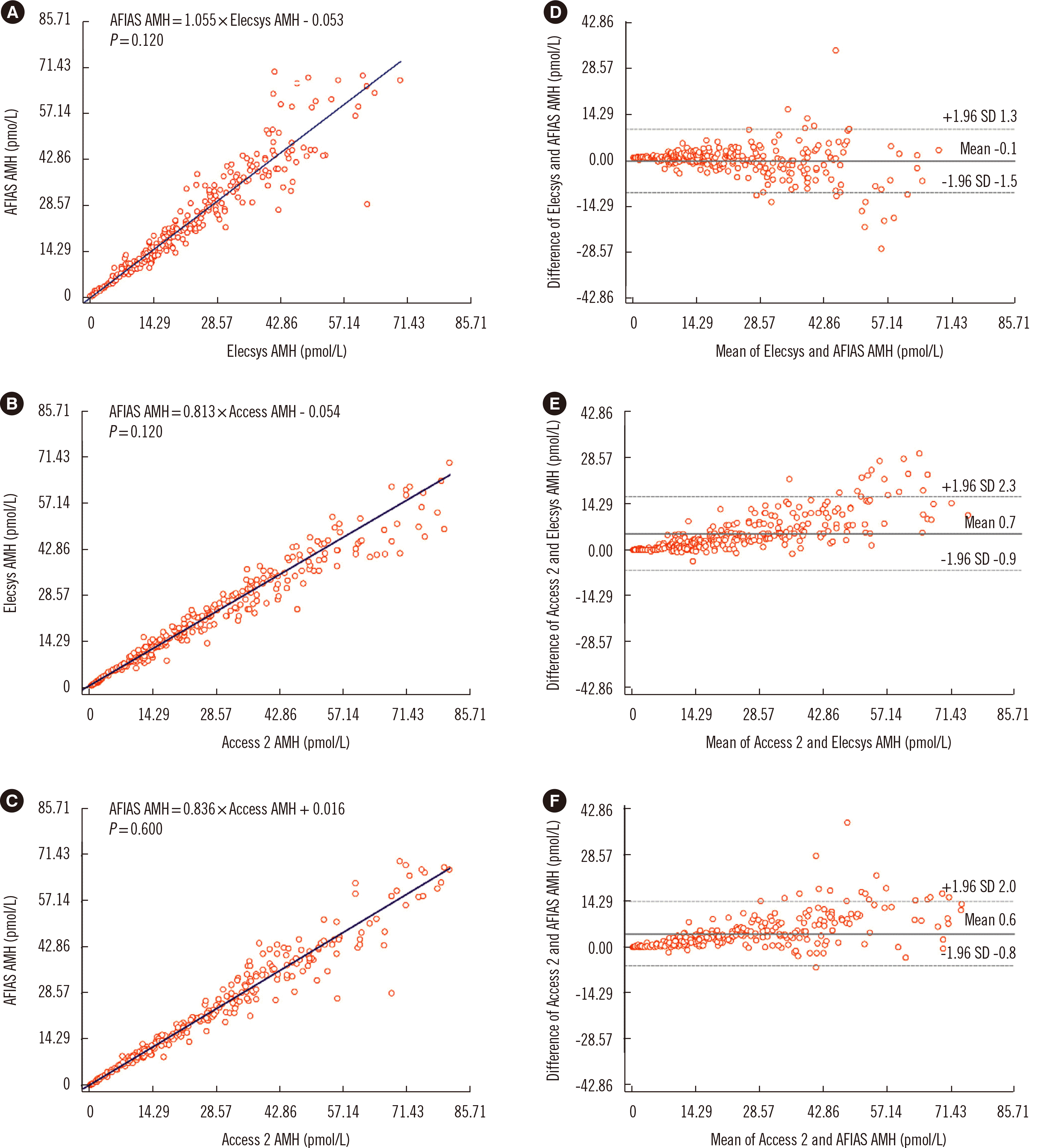
Table 1
Summary of linear and non-linear regression analyses of the AFIAS AMH assay results
Table 2
Repeatability and within-laboratory precision of the AFIAS AMH assay
Table 3
Nomogram of AMH levels (pmol/L) by age




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download