2. Nordmann P. 2014; Carbapenemase-producing Enterobacteriaceae: overview of a major public health challenge. Med Mal Infect. 44:51–6. DOI:
10.1016/j.medmal.2013.11.007. PMID:
24360201.

3. Walsh C. 2000; Molecular mechanisms that confer antibacterial drug resistance. Nature. 406:775–81. DOI:
10.1038/35021219. PMID:
10963607.

4. Lee M, Choi TJ. 2021; Antimicrobial resistance caused by KPC-2 encoded by promiscuous plasmids of the
Klebsiella pneumoniae ST307 strain. Ann Lab Med. 41:86–94. DOI:
10.3343/alm.2021.41.1.86. PMID:
32829583. PMCID:
PMC7443515.
5. Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. 2001; Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of
Klebsiella pneumoniae. Antimicrob Agents Chemother. 45:1151–61. DOI:
10.1128/AAC.45.4.1151-1161.2001. PMID:
11257029. PMCID:
PMC90438.
6. Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, et al. 2009; Characterization of a new metallo-beta-lactamase gene,
bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in
Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 53:5046–54. DOI:
10.1128/AAC.00774-09. PMID:
19770275. PMCID:
PMC2786356.
8. Poirel L, Héritier C, Tolün V, Nordmann P. 2004; Emergence of oxacillinase-mediated resistance to imipenem in
Klebsiella pneumoniae. Antimicrob Agents Chemother. 48:15–22. DOI:
10.1128/AAC.48.1.15-22.2004. PMID:
14693513. PMCID:
PMC310167.
9. Jeong SH, Kim HS, Kim JS, Shin DH, Kim HS, Park MJ, et al. 2016; Prevalence and molecular characteristics of carbapenemase-producing
Enterobacteriaceae from five hospitals in Korea. Ann Lab Med. 36:529–35. DOI:
10.3343/alm.2016.36.6.529. PMID:
27578505. PMCID:
PMC5011105.
10. Lee H, Yoon EJ, Kim D, Jeong SH, Won EJ, Shin JH, et al. 2018; Antimicrobial resistance of major clinical pathogens in South Korea, May 2016 to April 2017: first one-year report from Kor-GLASS. Euro Surveill. 23:1800047. DOI:
10.2807/1560-7917.ES.2018.23.42.1800047. PMID:
30352640. PMCID:
PMC6199864.

11. Song W, Hong SG, Yong D, Jeong SH, Kim HS, Kim HS, et al. 2015; Combined use of the modified Hodge test and carbapenemase inhibition test for detection of carbapenemase-producing
Enterobacteriaceae and metallo-beta-lactamase-producing
Pseudomonas spp. Ann Lab Med. 35:212–9. DOI:
10.3343/alm.2015.35.2.212.
12. Jeong S, Kim JO, Jeong SH, Bae IK, Song W. 2015; Evaluation of peptide nucleic acid-mediated multiplex real-time PCR kits for rapid detection of carbapenemase genes in gram-negative clinical isolates. J Microbiol Methods. 113:4–9. DOI:
10.1016/j.mimet.2015.03.019. PMID:
25819308.

13. CLSI. 2016. Performance standards for antimicrobial susceptibility testing. CLSI M100S. 27th ed. Clinical and Laboratory Standards Institute;Wayne, PA:
15. Song W, Park MJ, Jeong S, Shin DH, Kim JS, Kim HS, et al. 2020; Rapid identification of OXA-48-like, KPC, NDM, and VIM carbapenemase-producing
Enterobacteriaceae from culture: evaluation of the RESIST-4 O.K.N.V. multiplex lateral flow assay. Ann Lab Med. 40:259–63. DOI:
10.3343/alm.2020.40.3.259. PMID:
31858767. PMCID:
PMC6933055.
16. Nordmann P, Poirel L. 2014; The difficult-to-control spread of carbapenemase producers among
Enterobacteriaceae worldwide. Clin Microbiol Infect. 20:821–30. DOI:
10.1111/1469-0691.12719. PMID:
24930781.
17. Rhee JY, Park YK, Shin JY, Choi JY, Lee MY, Peck KR, et al. 2010; KPC-producing extreme drug-resistant
Klebsiella pneumoniae isolate from a patient with diabetes mellitus and chronic renal failure on hemodialysis in South Korea. Antimicrob Agents Chemother. 54:2278–9. DOI:
10.1128/AAC.00011-10. PMID:
20211897. PMCID:
PMC2863605.
18. Hong SK, Yong D, Kim K, Hong SS, Hong SG, Khosbayar T, et al. 2013; First outbreak of KPC-2-producing
Klebsiella pneumoniae sequence type 258 in a hospital in South Korea. J Clin Microbiol. 51:3877–9. DOI:
10.1128/JCM.01730-13. PMID:
24006005. PMCID:
PMC3889788.
19. Kim MN, Yong D, An D, Chung HS, Woo JH, Lee K, et al. 2012; Nosocomial clustering of NDM-1-producing
Klebsiella pneumoniae sequence type 340 strains in four patients at a South Korean tertiary care hospital. J Clin Microbiol. 50:1433–6. DOI:
10.1128/JCM.06855-11. PMID:
22259206. PMCID:
PMC3318568.
20. Yoo JS, Kim HM, Koo HS, Yang JW, Yoo JI, Kim HS, et al. 2013; Nosocomial transmission of NDM-1-producing
Escherichia coli ST101 in a Korean hospital. J Antimicrob Chemother. 68:2170–2. DOI:
10.1093/jac/dkt126. PMID:
23696618.
21. Sekirov I, Croxen MA, Ng C, Azana R, Chang Y, Mataseje L, et al. 2016; Epidemiologic and genotypic review of carbapenemase-producing organisms in British Columbia, Canada, between 2008 and 2014. J Clin Microbiol. 54:317–27. DOI:
10.1128/JCM.02289-15. PMID:
26607987. PMCID:
PMC4733174.

22. Jeong SH, Lee KM, Lee J, Bae IK, Kim JS, Kim HS, et al. 2015; Clonal and horizontal spread of the
blaOXA-232 gene among
Enterobacteriaceae in a Korean hospital. Diagn Microbiol Infect Dis. 82:70–2. DOI:
10.1016/j.diagmicrobio.2015.02.001. PMID:
25702524.
23. Poirel L, Potron A, Nordmann P. 2012; OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 67:1597–606. DOI:
10.1093/jac/dks121. PMID:
22499996.

25. Lee JY, Park JY, Kim JH, Lee YH, Yang HY, Yoo JS. 2017; Outbreak of imipenemase-1-producing carbapenem-resistant
Klebsiella pneumoniae in an intensive care unit. Korean J Crit Care Med. 32:29–38. DOI:
10.4266/kjccm.2016.00731. PMID:
31723613. PMCID:
PMC6786743.
26. Lauretti L, Riccio ML, Mazzariol A, Cornaglia G, Amicosante G, Fontana R, et al. 1999; Cloning and characterization of
blaVIM, a new integron-borne metallo-beta-lactamase gene from a
Pseudomonas aeruginosa clinical isolate. Antimicrob Agents Chemother. 43:1584–90. DOI:
10.1128/AAC.43.7.1584. PMID:
10390207. PMCID:
PMC89328.
27. Hong DJ, Bae IK, Jang IH, Jeong SH, Kang HK, Lee K. 2015; Epidemiology and characteristics of metallo-beta-lactamase-producing
Pseudomonas aeruginosa. Infect Chemother. 47:81–97. DOI:
10.3947/ic.2015.47.2.81.
28. Tseng IL, Liu YM, Wang SJ, Yeh HY, Hsieh CL, Lu HL, et al. 2015; Emergence of carbapenemase producing
Klebsiella pneumonia and spread of KPC-2 and KPC-17 in Taiwan: A nationwide study from 2011 to 2013. PLoS One. 10:e0138471. DOI:
10.1371/journal.pone.0138471. PMID:
26384242. PMCID:
PMC4575059.
29. Endimiani A, Perez F, Bonomo RA. 2008; Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther. 6:805–24. DOI:
10.1586/14787210.6.6.805. PMID:
19053894. PMCID:
PMC2633657.

30. Sader HS, Fritsche TR, Jones RN. 2005; Potency and spectrum trends for cefepime tested against 65746 clinical bacterial isolates collected in North American medical centers: results from the SENTRY Antimicrobial Surveillance Program (1998-2003). Diagn Microbiol Infect Dis. 52:265–73. DOI:
10.1016/j.diagmicrobio.2005.02.003. PMID:
16105569.

31. Ji S, Lv F, Du X, Wei Z, Fu Y, Mu X, et al. 2015; Cefepime combined with amoxicillin/clavulanic acid: a new choice for the KPC-producing
K. pneumoniae infection. Int J Infect Dis. 38:108–14. DOI:
10.1016/j.ijid.2015.07.024. PMID:
26255892.
32. Bartolini A, Basso M, Franchin E, Menegotto N, Ferrari A, De Canale E, et al. 2017; Prevalence, molecular epidemiology and intra-hospital acquisition of
Klebsiella pneumoniae strains producing carbapenemases in an Italian teaching hospital from January 2015 to September 2016. Int J Infect Dis. 59:103–9. DOI:
10.1016/j.ijid.2017.04.007. PMID:
28412407.
33. Fuster B, Tormo N, Salvador C, Gimeno C. 2020; Detection of two simultaneous outbreaks of
Klebsiella pneumoniae coproducing OXA-48 and NDM-1 carbapenemases in a tertiary-care hospital in Valencia, Spain. New Microbes New Infect. 34:100660. DOI:
10.1016/j.nmni.2020.100660. PMID:
32194965. PMCID:
PMC7075970.
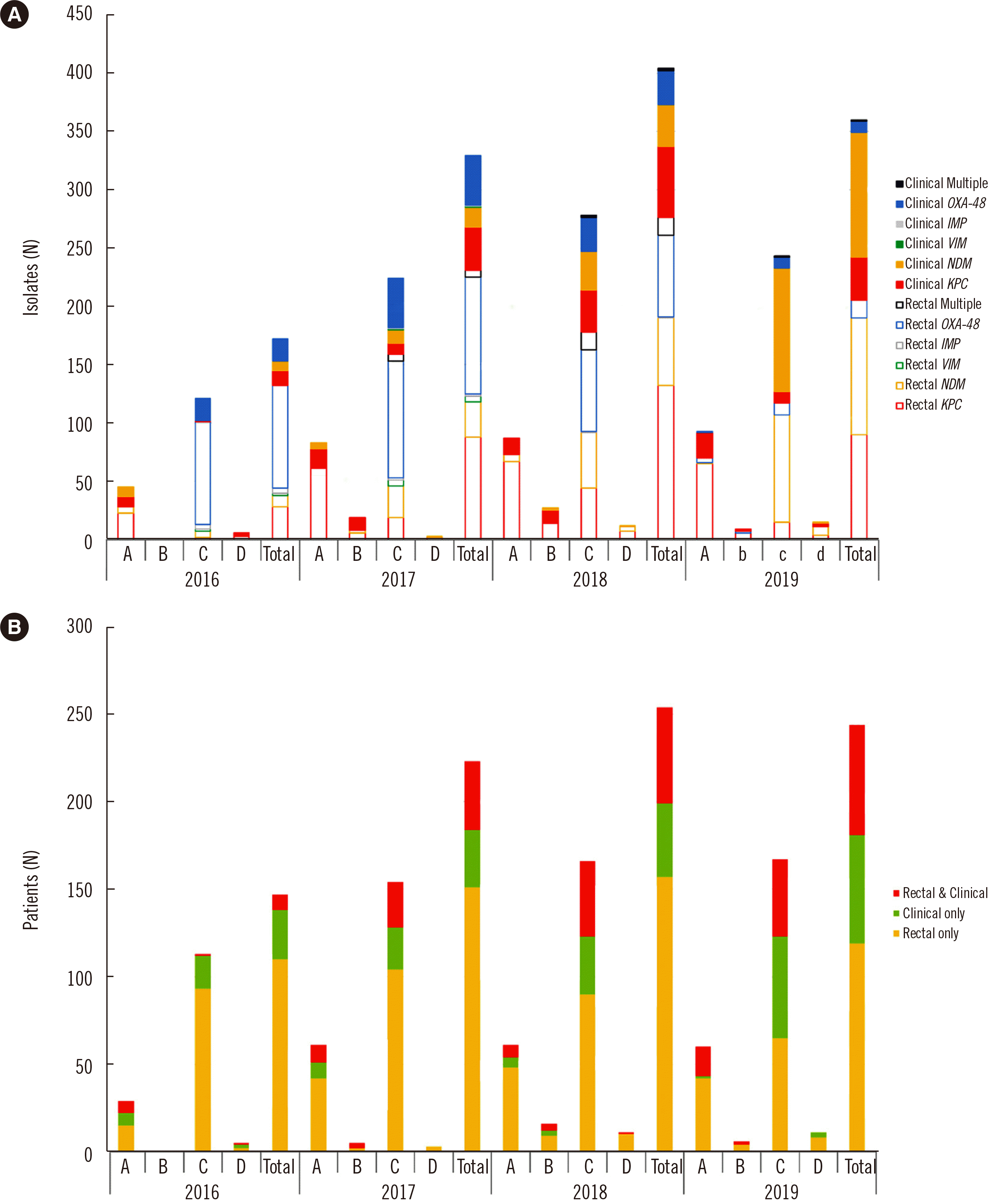
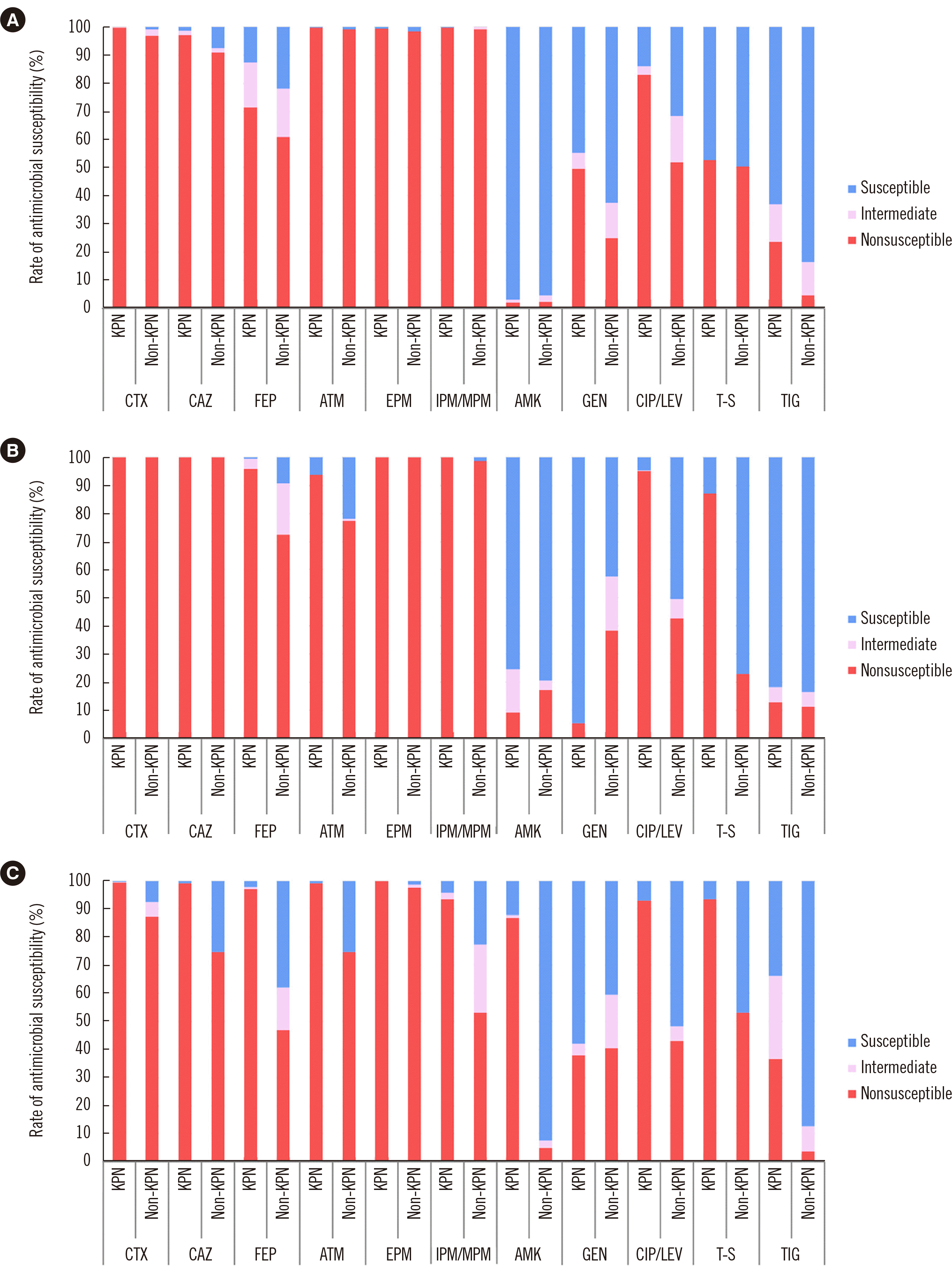
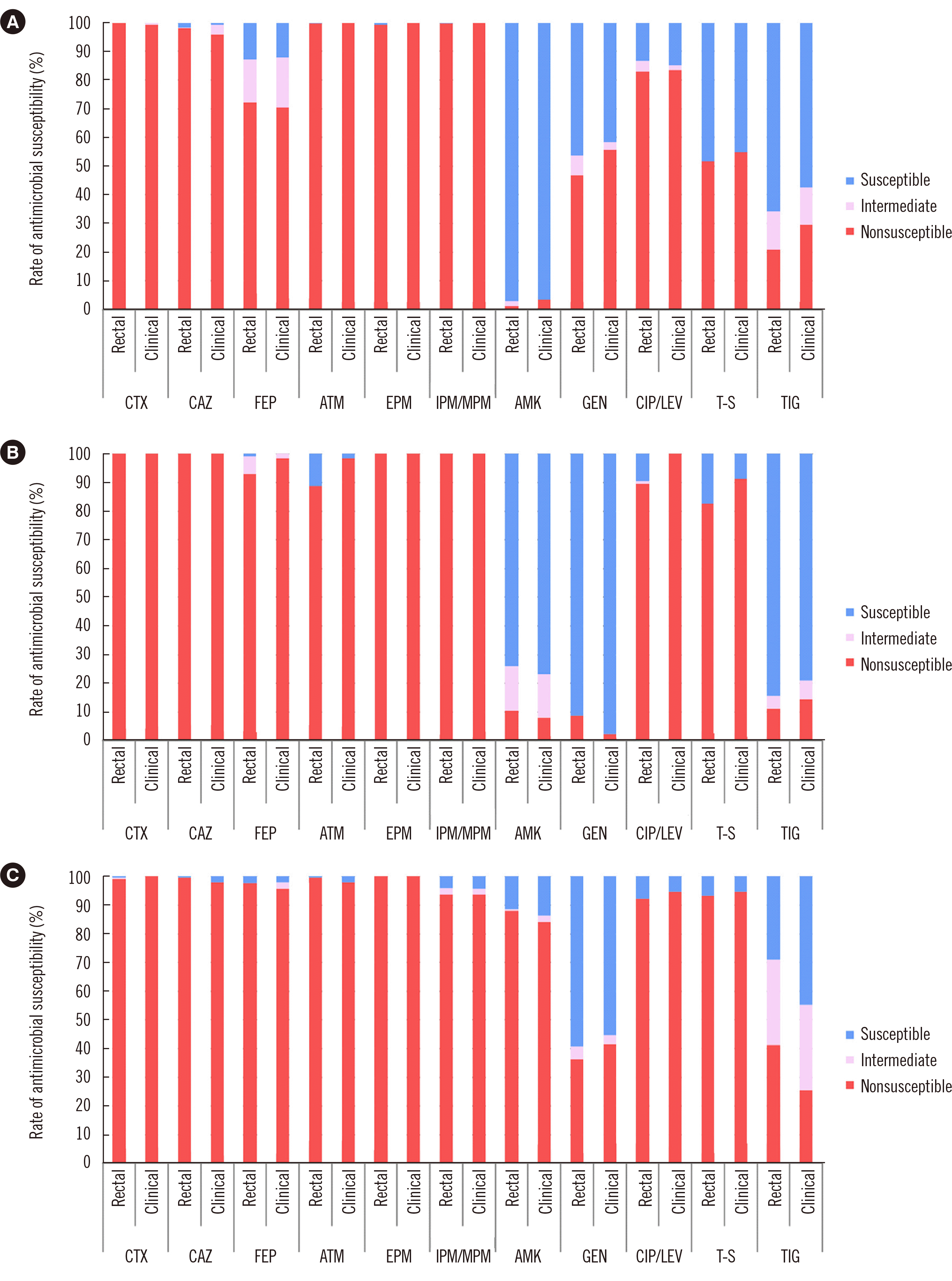




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download