1. Wu AH. 2006; A selected history and future of immunoassay development and applications in clinical chemistry. Clin Chim Acta. 369:119–24. DOI:
10.1016/j.cca.2006.02.045. PMID:
16701599.

2. Sturgeon CM, Viljoen A. 2011; Analytical error and interference in immunoassay: minimizing risk. Ann Clin Biochem. 48:418–32. DOI:
10.1258/acb.2011.011073. PMID:
21750113.

3. Köhler G, Milstein C. 1975; Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 256:495–7. DOI:
10.1038/256495a0. PMID:
1172191.

4. Tate J, Ward G. 2004; Interferences in immunoassay. Clin Biochem Rev. 25:105–20. PMID:
18458713. PMCID:
PMC1904417.
5. Sturgeon CM. 2013; External quality assessment of hormone determinations. Best Pract Res Clin Endocrinol Metab. 27:803–22. DOI:
10.1016/j.beem.2013.08.009. PMID:
24275192.

6. Darwish IA. 2006; Immunoassay methods and their applications in pharmaceutical analysis: basic methodology and recent advances. Int J Biomed Sci. 2:217–35. PMID:
23674985. PMCID:
PMC3614608.
7. Caruso B, Bovo C, Guidi GC. 2020; Causes of preanalytical interferences on laboratory immunoassays-A critical review. EJIFCC. 31:70–84. PMID:
32256291. PMCID:
PMC7109499.
9. Long N, Nguyen L, Stevermer J. 2015; PURLS: It's time to reconsider early-morning testosterone tests. J Fam Pract. 64:418–9. PMID:
26324959. PMCID:
PMC4501456.
11. Owen LJ, Monaghan PJ, Armstrong A, Keevil BG, Higham C, Salih Z, et al. 2019; Oestradiol measurement during fulvestrant treatment for breast cancer. Br J Cancer. 120:404–6. DOI:
10.1038/s41416-019-0378-9. PMID:
30679781. PMCID:
PMC6461991.

12. Mandic S, Kratzsch J, Mandic D, Debeljak Z, Lukic I, Horvat V, et al. 2017; Falsely elevated serum oestradiol due to exemestane therapy. Ann Clin Biochem. 54:402–5. DOI:
10.1177/0004563216674031. PMID:
27687081.

13. Valdes R, Jortani SA. 2002; Unexpected suppression of immunoassay results by cross-reactivity: now a demonstrated cause for concern. Clin Chem. 48:405–6. DOI:
10.1093/clinchem/48.3.405. PMID:
11861431.

14. Lopez AG, Fraissinet F, Lefebvre H, Brunel V, Ziegler F. 2019; Pharmacological and analytical interference in hormone assays for diagnosis of adrenal incidentaloma. Ann Endocrinol (Paris). 80:250–8. DOI:
10.1016/j.ando.2018.11.006. PMID:
31445667.

15. Groenestege WM, Bui HN, ten Kate J, Menheere PP, Oosterhuis WP, Vader HL, et al. 2012; Accuracy of first and second generation testosterone assays and improvement through sample extraction. Clin Chem. 58:1154–6. DOI:
10.1373/clinchem.2011.181735. PMID:
22539807.

16. Benton SC, Nuttall M, Nardo L, Laing I. 2011; Measured dehydroepiandrosterone sulfate positively influences testosterone measurement in unextracted female serum: comparison of 2 immunoassays with testosterone measured by LC-MS. Clin Chem. 57:1074–5. DOI:
10.1373/clinchem.2010.158600. PMID:
21444736.

18. Paisley AN, Hayden K, Ellis A, Anderson J, Wieringa G, Trainer PJ. 2007; Pegvisomant interference in GH assays results in underestimation of GH levels. Eur J Endocrinol. 156:315–9. DOI:
10.1530/eje.1.02341. PMID:
17322491.

19. Manolopoulou J, Alami Y, Petersenn S, Schopohl J, Wu Z, Strasburger CJ, et al. 2012; Automated 22-kD growth hormone-specific assay without interference from Pegvisomant. Clin Chem. 58:1446–56. DOI:
10.1373/clinchem.2012.188128. PMID:
22908135.

20. Raff H, Findling JW. 1989; A new immunoradiometric assay for corticotropin evaluated in normal subjects and patients with Cushing's syndrome. Clin Chem. 35:596–600. DOI:
10.1093/clinchem/35.4.596. PMID:
2539271.

21. Woeber KA, White A, Kurtz TW. 2014; Recurrent pituitary macroadenoma with increased plasma ACTH precursors that cross-react in a commonly used ACTH immunoassay. Endocr Pract. 20:94–6. DOI:
10.4158/EP13299.CO. PMID:
24126231.

22. Parfitt C, Church D, Armston A, Couchman L, Evans C, Wark G, et al. 2015; Commercial insulin immunoassays fail to detect commonly prescribed insulin analogues. Clin Biochem. 48:1354–7. DOI:
10.1016/j.clinbiochem.2015.07.017. PMID:
26171976.

23. Dayaldasani A, Rodríguez Espinosa M, Ocón Sánchez P, Pérez Valero V. 2015; Cross-reactivity of insulin analogues with three insulin assays. Ann Clin Biochem. 52:312–8. DOI:
10.1177/0004563214551613. PMID:
25172526.

24. Giurgea I, Ulinski T, Touati G, Sempoux C, Mochel F, Brunelle F, et al. 2005; Factitious hyperinsulinism leading to pancreatectomy: severe forms of Munchausen syndrome by proxy. Pediatrics. 116:e145–8. DOI:
10.1542/peds.2004-2331. PMID:
15995015.

25. Drees JC, Stone JA, Reamer CR, Arboleda VE, Huang K, Hrynkow J, et al. 2014; Falsely undetectable TSH in a cohort of South Asian euthyroid patients. J Clin Endocrinol Metab. 99:1171–9. DOI:
10.1210/jc.2013-2092. PMID:
24423284.

27. Haddad RA, Giacherio D, Barkan AL. 2019; Interpretation of common endocrine laboratory tests: technical pitfalls, their mechanisms and practical considerations. Clin Diabetes Endocrinol. 5:12. DOI:
10.1186/s40842-019-0086-7. PMID:
31367466. PMCID:
PMC6657094.

28. Frieze TW, Mong DP, Koops MK. 2002; "Hook effect" in prolactinomas: case report and review of literature. Endocr Pract. 8:296–303. DOI:
10.4158/EP.8.4.296. PMID:
12173917.

29. Bolstad N, Warren DJ, Nustad K. 2013; Heterophilic antibody interference in immunometric assays. Best Pract Res Clin Endocrinol Metab. 27:647–61. DOI:
10.1016/j.beem.2013.05.011. PMID:
24094636.

30. Ismail AA. 2014; Identifying and reducing potentially wrong immunoassay results even when plausible and "not-unreasonable". Adv Clin Chem. 66:241–94. DOI:
10.1016/B978-0-12-801401-1.00007-4. PMID:
25344990.

31. Favresse J, Burlacu MC, Maiter D, Gruson D. 2018; Interferences with thyroid function immunoassays: clinical implications and detection algorithm. Endocr Rev. 39:830–50. DOI:
10.1210/er.2018-00119. PMID:
29982406.

33. Bergman D, Larsson A, Hansson-Hamlin H, Åhlén E, Holst BS. 2019; Characterization of canine anti-mouse antibodies highlights that multiple strategies are needed to combat immunoassay interference. Sci Rep. 9. DOI:
10.1038/s41598-019-51228-3. PMID:
31601945. PMCID:
PMC6787031.

34. Boscato LM, Stuart MC. 1988; Heterophilic antibodies: a problem for all immunoassays. Clin Chem. 34:27–33. DOI:
10.1093/clinchem/34.1.27. PMID:
3338181.

35. Hattori N, Ishihara T, Shimatsu A. 2016; Variability in the detection of macro TSH in different immunoassay systems. Eur J Endocrinol. 174:9–15. DOI:
10.1530/EJE-15-0883. PMID:
26438715.

36. Giovanella L, Ghelfo A. 2007; Undetectable serum thyroglobulin due to negative interference of heterophile antibodies in relapsing thyroid carcinoma. Clin Chem. 53:1871–2. DOI:
10.1373/clinchem.2007.093229. PMID:
17885144.

37. Ramaeker D, Brannian J, Egland K, McCaul K, Hansen K. 2008; When is elevated testosterone not testosterone? When it is an immunoassay interfering antibody. Fertil Steril. 90:886–8. DOI:
10.1016/j.fertnstert.2007.07.1295.

38. Brugts MP, Luermans JG, Lentjes EG, Van Trooyen-van Vrouwerff NJ, Van der Horst FA, Slee PH, et al. 2009; Heterophilic antibodies may be a cause of falsely low total IGF1 levels. Eur J Endocrinol. 161:561–5. DOI:
10.1530/EJE-09-0316. PMID:
19608715.

39. Saleem M, Lewis JG, Florkowski CM, Mulligan GP, George PM, Hale P. 2009; A patient with pseudo-Addison's disease and falsely elevated thyroxine due to interference in serum cortisol and free thyroxine immunoassays by two different mechanisms. Ann Clin Biochem. 46:172–5. DOI:
10.1258/acb.2008.008224. PMID:
19225029.

40. Gulbahar O, Konca Degertekin C, Akturk M, Yalcin MM, Kalan I, Atikeler GF, et al. 2015; A case with immunoassay interferences in the measurement of multiple hormones. J Clin Endocrinol Metab. 100:2147–53. DOI:
10.1210/jc.2014-4023. PMID:
25897621.

41. García-González E, Aramendía M, Álvarez-Ballano D, Trincado P, Rello L. 2015; Serum sample containing endogenous antibodies interfering with multiple hormone immunoassays. Laboratory strategies to detect interference. Pract Lab Med. 4:1–10. DOI:
10.1016/j.plabm.2015.11.001. PMID:
28856186. PMCID:
PMC5574524.

42. Oei AL, Sweep FC, Massuger LF, Olthaar AJ, Thomas CM. 2008; Transient human anti-mouse antibodies (HAMA) interference in CA 125 measurements during monitoring of ovarian cancer patients treated with murine monoclonal antibody. Gynecol Oncol. 109:199–202. DOI:
10.1016/j.ygyno.2008.01.025. PMID:
18304620.

43. Kazmierczak SC, Catrou PG, Briley KP. 2000; Transient nature of interference effects from heterophile antibodies: examples of interference with cardiac marker measurements. Clin Chem Lab Med. 38:33–9. DOI:
10.1515/CCLM.2000.006. PMID:
10774959.

44. Berglund Land Holmberg NG. 1989; Heterophilic antibodies against rabbit serum causing falsely elevated gonadotropin levels. Acta Obstet Gynecol Scand. 68:377–8. DOI:
10.3109/00016348909028676. PMID:
2515728.

47. Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, et al. 2020; Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 27:1. DOI:
10.1186/s12929-019-0592-z. PMID:
31894001. PMCID:
PMC6939334.

48. Castinetti F, Albarel F, Archambeaud F, Bertherat J, Bouillet B, Buffier P, et al. 2019; French endocrine society guidance on endocrine side effects of immunotherapy. Endocr Relat Cancer. 26:G1–18. DOI:
10.1530/ERC-18-0320. PMID:
30400055. PMCID:
PMC6347286.

49. Lahlou N, Raverot V. 2018; Expert opinions on endocrine toxicity induced by new anticancer therapies: precautions to be taken in performing and interpreting hormonal assays under immunotherapy. Ann Endocrinol (Paris). 79:550–4. DOI:
10.1016/j.ando.2018.07.004. PMID:
30149892.

50. Koshida S, Asanuma K, Kuribayashi K, Goto M, Tsuji N, Kobayashi D, et al. 2010; Prevalence of human anti-mouse antibodies (HAMAs) in routine examinations. Clin Chim Acta. 411:391–4. DOI:
10.1016/j.cca.2009.12.006. PMID:
20006593.

51. Emerson JF, Lai KKY. 2013; Endogenous antibody interferences in immunoassays. Lab Med. 44:69–73. DOI:
10.1309/LMMURCFQHKSB5YEC.

52. Jonklaas J. 2016; TSH-free thyroxine discordance in an athyreotic patient during ipiluminab and nivoluminab therapy. AACE Clin Case Rep. 2:e296–301. DOI:
10.4158/EP15975.CR.

53. Kim D, Hong N, Cho Y, Lee SG, Rhee Y. 2020; Heterophile antibody interference associated with natural killer cell therapy. Endocr J. 67:1187–92. DOI:
10.1507/endocrj.EJ20-0349. PMID:
32713865.

54. Holm BE, Sandhu N, Tronstrøm J, Lydolph M, Trier NH, Houen G. 2015; Species cross-reactivity of rheumatoid factors and implications for immunoassays. Scand J Clin Lab Invest. 75:51–63. DOI:
10.3109/00365513.2014.965738. PMID:
25347362.

55. Astarita G, Gutiérrez S, Kogovsek N, Mormandi E, Otero P, Calabrese C, et al. 2015; False positive in the measurement of thyroglobulin induced by rheumatoid factor. Clin Chim Acta. 447:43–6. DOI:
10.1016/j.cca.2015.04.039. PMID:
25979693.

56. Mongolu S, Armston AE, Mozley E, Nasruddin A. 2016; Heterophilic antibody interference affecting multiple hormone assays: is it due to rheumatoid factor? Scand J Clin Lab Invest. 76:240–2. DOI:
10.3109/00365513.2016.1143113. PMID:
26924790.

57. Cheng X, Guo X, Chai X, Hu Y, Lian X, Zhang G. 2021; Heterophilic antibody interference with TSH measurement on different immunoassay platforms. Clin Chim Acta. 512:63–5. DOI:
10.1016/j.cca.2020.11.018. PMID:
33285118.

58. Laudes M, Frohnert J, Ivanova K, Wandinger KP. 2019; PTH immunoassay interference due to human anti-mouse antibodies in a subject with obesity with normal parathyroid function. J Clin Endocrinol Metab. 104:5840–2. DOI:
10.1210/jc.2019-01321. PMID:
31411693.

59. Fruzzetti F, Palla G, Sbrana A, Simoncini T, Sessa MR. 2020; Anti-goat antibodies as a rare cause of high gonadotropin levels during menopausal transition. Gynecol Endocrinol. 36:938–40. DOI:
10.1080/09513590.2020.1780580. PMID:
33021135.

60. Aliberti L, Gagliardi I, Dorizzi RM, Pizzicotti S, Bondanelli M, Zatelli MC, et al. 2021; Hypeprolactinemia: still an insidious diagnosis. Endocrine. 72:928–31. DOI:
10.1007/s12020-020-02497-w. PMID:
32949349. PMCID:
PMC8159778.

61. Hattori N, Aisaka K, Shimatsu A. 2016; A possible cause of the variable detectability of macroprolactin by different immunoassay systems. Clin Chem Lab Med. 54:603–8. DOI:
10.1515/cclm-2015-0484. PMID:
26457779.

62. Overgaard M, Pedersen SM. 2017; Serum prolactin revisited: parametric reference intervals and cross platform evaluation of polyethylene glycol precipitation-based methods for discrimination between hyperprolactinemia and macroprolactinemia. Clin Chem Lab Med. 55:1744–53. DOI:
10.1515/cclm-2016-0902. PMID:
28236625.

63. Fahie-Wilson M, Smith TP. 2013; Determination of prolactin: the macroprolactin problem. Best Pract Res Clin Endocrinol Metab. 27:725–42. DOI:
10.1016/j.beem.2013.07.002. PMID:
24094642.

64. Hattori N, Ikekubo K, Nakaya Y, Kitagawa K, Inagaki C. 2005; Immunoglobulin G subclasses and prolactin (PRL) isoforms in macroprolactinemia due to anti-PRL autoantibodies. J Clin Endocrinol Metab. 90:3036–44. DOI:
10.1210/jc.2004-1600. PMID:
15687336.

65. Richa V, Rahul G, Sarika A. 2010; Macroprolactin; a frequent cause of misdiagnosed hyperprolactinemia in clinical practice. J Reprod Infertil. 11:161–7. PMID:
23926484. PMCID:
PMC3719302.
66. Fahie-Wilson M, Halsall D. 2008; Polyethylene glycol precipitation: proceed with care. Ann Clin Biochem. 45:233–5. DOI:
10.1258/acb.2008.007262. PMID:
18482908.

67. Barth JH, Lippiatt CM, Gibbons SG, Desborough RA. 2018; Observational studies on macroprolactin in a routine clinical laboratory. Clin Chem Lab Med. 56:1259–62. DOI:
10.1515/cclm-2018-0074. PMID:
29630507.

68. Webster R, Fahie-Wilson M, Barker P, Chatterjee VK, Halsall DJ. 2010; Immunoglobulin interference in serum follicle-stimulating hormone assays: autoimmune and heterophilic antibody interference. Ann Clin Biochem. 47:386–9. DOI:
10.1258/acb.2010.010044. PMID:
20511373.

69. Ohba K, Maekawa M, Iwahara K, Suzuki Y, Matsushita A, Sasaki S, et al. 2020; Abnormal thyroid hormone response to TRH in a case of macro-TSH and the cut-off value for screening cases of inappropriate TSH elevation. Endocr J. 67:125–30. DOI:
10.1507/endocrj.EJ19-0320. PMID:
31645528.

70. Loh TP, Kao SL, Halsall DJ, Toh SA, Chan E, Ho SC, et al. 2012; Macro-thyrotropin: a case report and review of literature. J Clin Endocrinol Metab. 97:1823–8. DOI:
10.1210/jc.2011-3490. PMID:
22466337.

71. Rix M, Laurberg P, Porzig C, Kristensen SR. 2011; Elevated thyroid-stimulating hormone level in a euthyroid neonate caused by macro thyrotropin-IgG complex. Acta Paediatr. 100:e135–7. DOI:
10.1111/j.1651-2227.2011.02212.x. PMID:
21352360.

72. Halsall DJ, Fahie-Wilson MN, Hall SK, Barker P, Anderson J, Gama R, et al. 2006; Macro thyrotropin-IgG complex causes factitious increases in thyroid-stimulating hormone screening tests in a neonate and mother. Clin Chem. 52:1968–9. DOI:
10.1373/clinchem.2006.071050. PMID:
16998119.

73. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2016; 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 26:1–133. DOI:
10.1089/thy.2015.0020. PMID:
26462967. PMCID:
PMC4739132.

74. Spencer C, LoPresti J, Fatemi S. 2014; How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr Opin Endocrinol Diabetes Obes. 21:394–404. DOI:
10.1097/MED.0000000000000092. PMID:
25122493. PMCID:
PMC4154792.

75. Giovanella L, Feldt-Rasmussen U, Verburg FA, Grebe SK, Plebani M, Clark PM. 2015; Thyroglobulin measurement by highly sensitive assays: focus on laboratory challenges. Clin Chem Lab Med. 53:1301–14. DOI:
10.1515/cclm-2014-0813. PMID:
25355247.

76. Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. 2009; Autoimmune forms of hypoglycemia. Medicine (Baltimore). 88:141–53. DOI:
10.1097/MD.0b013e3181a5b42e. PMID:
19440117.

77. Ismail AA. 2016; The insulin autoimmune syndrome (IAS) as a cause of hypoglycaemia: an update on the pathophysiology, biochemical investigations and diagnosis. Clin Chem Lab Med. 54:1715–24. DOI:
10.1515/cclm-2015-1255. PMID:
27071154.

78. Lamy PJ, Sault C, Renard E. 2016; High fasting serum insulin level due to autoantibody interference in insulin immunoassay discloses autoimmune insulin syndrome: a case report. Ann Biol Clin (Paris). 74:490–4. DOI:
10.1684/abc.2016.1168. PMID:
27492703.

79. Piketty ML, Brabant S, Souberbielle JC, Maruani G, Audrain C, Rothenbuhler A, et al. 2020; FGF23 measurement in burosumab-treated patients: an emerging treatment may induce a new analytical interference. Clin Chem Lab Med. 58:e267–9. DOI:
10.1515/cclm-2020-0460. PMID:
32653872.

80. Lee MN, Lee SY, Hur KY, Park HD. 2017; Thyroxine (T4) autoantibody interference of free T4 concentration measurement in a patient with Hashimotós thyroiditis. Ann Lab Med. 37:169–71. DOI:
10.3343/alm.2017.37.2.169.
81. Zouwail SA, O'Toole AM, Clark PM, Begley JP. 2008; Influence of thyroid hormone autoantibodies on 7 thyroid hormone assays. Clin Chem. 54:927–8. DOI:
10.1373/clinchem.2007.099770. PMID:
18443182.

82. Massart C, Elbadii S, Gibassier J, Coignard V, Rasandratana A. 2009; Anti-thyroxine and anti-triiodothyronine antibody interferences in one-step free triiodothyronine and free thyroxine immunoassays. Clin Chim Acta. 401:175–6. DOI:
10.1016/j.cca.2008.11.001. PMID:
19028480.

83. Beato-Víbora PI, Alejo-González S. 2016; Avoiding misdiagnosis due to antibody interference with serum free thyroxin. Int J Endocrinol Metab. 15:e37792. DOI:
10.5812/ijem.37792. PMID:
28835757. PMCID:
PMC5554610.

84. Srichomkwun P, Scherberg NH, Jakšić J, Refetoff S. 2017; Diagnostic dilemma in discordant thyroid function tests due to thyroid hormone autoantibodies. AACE Clin Case Rep. 3:e22–5. DOI:
10.4158/EP151142.CR. PMID:
28078322. PMCID:
PMC5222615.

86. Colucci R, Lotti F, Arunachalam M, Lotti T, Dragoni F, Benvenga S, et al. 2015; Correlation of serum thyroid hormones autoantibodies with self-reported exposure to thyroid disruptors in a group of nonsegmental vitiligo patients. Arch Environ Contam Toxicol. 69:181–90. DOI:
10.1007/s00244-015-0138-7. PMID:
25700983.

87. Papo T, Oksenhendler E, Izembart M, Leger A, Clauvel JP. 1992; Antithyroid hormone antibodies induced by interferon-alpha. J Clin Endocrinol Metab. 75:1484–6. DOI:
10.1210/jcem.75.6.1464652. PMID:
1464652.

88. Shimon I, Pariente C, Shlomo-David J, Grossman Z, Sack J. 2003; Transient elevation of triiodothyronine caused by triiodothyronine autoantibody associated with acute Epstein-Barr-virus infection. Thyroid. 13:211–5. DOI:
10.1089/105072503321319530. PMID:
12699597.

89. Henry JG, Sobki S, Arafat N. 1996; Interference by biotin therapy on measurement of TSH and FT4 by enzyme immunoassay on Boehringer Mannheim ES700 analyser. Ann Clin Biochem. 33:162–3. DOI:
10.1177/000456329603300214. PMID:
8729729.
90. Trambas C. Dasgupta A, Sepulveda JL, editors. 2019. Biotin interference in clinical laboratory tests: sporadic problem or a serious clinical issue? Accurate results in the clinical laboratory. 2nd ed. Elsevier;Amsterdam: p. 83–97. DOI:
10.1016/B978-0-12-813776-5.00008-X.
91. Barbesino G. 2016; Misdiagnosis of Graves' disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid. 26:860–3. DOI:
10.1089/thy.2015.0664.

92. Elston MS, Sehgal S, Du Toit S, Yarndley T, Conaglen JV. 2016; Factitious Graves' disease due to biotin immunoassay interference-A case and review of the literature. J Clin Endocrinol Metab. 101:3251–5. DOI:
10.1210/jc.2016-1971. PMID:
27362288.

94. Piketty ML, Polak M, Flechtner I, Gonzales-Briceño L, Souberbielle JC. 2017; False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences. Clin Chem Lab Med. 55:780–8. DOI:
10.1515/cclm-2016-0606. PMID:
27732554.

95. Carter GD, Berry J, Cavalier E, Durazo-Arvizu R, Gunter E, Jones G, et al. 2020; Biotin supplementation causes erroneous elevations of results in some commercial serum 25-hydroxyvitamin D (25OHD) assays. J Steroid Biochem Mol Biol. 200:105639. DOI:
10.1016/j.jsbmb.2020.105639. PMID:
32084550.

96. Plasse RA, Olson SW, Yuan CM, Nee R. 2020; Biotin supplement interference with immunoassays for parathyroid hormone and 25-hydroxyvitamin D in a patient with metabolic bone disease on maintenance hemodialysis. Clin Kidney J. 13:710–2. DOI:
10.1093/ckj/sfz134. PMID:
32905171. PMCID:
PMC7467599.

97. Stieglitz HM, Korpi-Steiner N, Katzman B, Mersereau JE, Styner M. 2018; Suspected testosterone-producing tumor in a patient taking biotin supplements. J Endocr Soc. 2:563–9. DOI:
10.1210/js.2018-00069. PMID:
29942920. PMCID:
PMC6007242.

98. Bizzarri C, Giannone GA, Gervasoni J, Benedetti S, Albanese F, Dello Strologo L, et al. 2020; Aug. 25. Unusual presentation of a Denys-Drash syndrome girl with undisclosed assumption of biotin. J Clin Res Pediatr Endocrinol. doi: 10.4274/jcrpe.galenos.2020.2020.0064. Online ahead of print. DOI:
10.4274/jcrpe.galenos.2020.2020.0064. PMID:
32840097.
99. Meany DL, Jan De Beur SM, Bill MJ, Sokoll LJ. 2009; A case of renal osteodystrophy with unexpected serum intact parathyroid hormone concentrations. Clin Chem. 55:1737–9. DOI:
10.1373/clinchem.2008.121921. PMID:
19717659.

100. Koehler VF, Mann U, Nassour A, Mann WA. 2018; Fake news? Biotin interference in thyroid immunoassays. Clin Chim Acta. 484:320–2. DOI:
10.1016/j.cca.2018.05.053. PMID:
29856977.

101. Ranaivosoa MK, Ganel S, Agin A, Romain S, Parent X, Reix N. 2017; Chronic kidney failure and biotin: A combination inducing unusual results in thyroid and parathyroid investigations, report of 2 cases. Nephrol Ther. 13:553–8. DOI:
10.1016/j.nephro.2017.02.016. PMID:
29133077.
102. Feldt MM. 2020; Delayed diagnosis of congenital hypothyroidism in a child with trisomy 21 and biotinidase deficiency and successful use of levothyroxine sodium oral solution. Case Rep Endocrinol. 2020:8883969. DOI:
10.1155/2020/8883969. PMID:
33425403. PMCID:
PMC7773459.

103. John JJ, Cooley V, Lipner SR. 2019; Assessment of biotin supplementation among patients in an outpatient dermatology clinic. J Am Acad Dermatol. 81:620–1. DOI:
10.1016/j.jaad.2018.12.045. PMID:
30630025.

104. Lipner SR. 2020; Update on biotin therapy in dermatology: time for a change. J Drugs Dermatol. 19:1264–5. DOI:
10.36849/JDD.2020.4946. PMID:
33346513.

105. Soleymani T, Lo Sicco K, Shapiro J. 2017; The infatuation with biotin supplementation: is there truth behind its rising popularity? A Comparative analysis of clinical efficacy versus social popularity. J Drugs Dermatol. 16:496–500. PMID:
28628687.
107. Katzman BM, Lueke AJ, Donato LJ, Jaffe AS, Baumann NA. 2018; Prevalence of biotin supplement usage in outpatients and plasma biotin concentrations in patients presenting to the emergency department. Clin Biochem. 60:11–6. DOI:
10.1016/j.clinbiochem.2018.07.004. PMID:
30036510.

108. Trambas CM, Liu KC, Luu H, Louey W, Lynch C, Yen T, et al. 2019; Further assessment of the prevalence of biotin supplementation and its impact on risk. Clin Biochem. 65:64–5. DOI:
10.1016/j.clinbiochem.2019.01.004. PMID:
30677402.

109. Ijpelaar A, Beijers A, van Daal H, van den Ouweland JMW. 2020; Prevalence of detectable biotin in the Netherlands in relation to risk on immunoassay interference. Clin Biochem. 83:78–80. DOI:
10.1016/j.clinbiochem.2020.05.009. PMID:
32473152.

110. Sanders A, Gama R, Ashby H, Mohammed P. 2021; Biotin immunoassay interference: a UK-based prevalence study. Ann Clin Biochem. 58:66–9. DOI:
10.1177/0004563220961759. PMID:
32936669.
113. Cree BAC, Cutter G, Wolinsky JS, Freedman MS, Comi G, Giovannoni G, et al. 2020; Safety and efficacy of MD1003 (high-dose biotin) in patients with progressive multiple sclerosis (SPI2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 19:988–97. DOI:
10.1016/S1474-4422(20)30347-1. PMID:
33222767.
114. Wolf B. 2019; High doses of biotin can interfere with immunoassays that use biotin-strept(avidin) technologies: implications for individuals with biotin-responsive inherited metabolic disorders. Mol Genet Metab. 127:321–4. DOI:
10.1016/j.ymgme.2019.07.003. PMID:
31320189.

115. Evans N, Yates J, Tobin J, McGill J, Huynh T. 2018; Immunoassay interference secondary to therapeutic high-dose biotin: A paediatric case report. J Paediatr Child Health. 54:572–5. DOI:
10.1111/jpc.13857. PMID:
29405469.

116. Bülow Pedersen I, Laurberg P. 2016; Biochemical hyperthyroidism in a newborn baby caused by assay interaction from biotin intake. Eur Thyroid J. 5:212–5. DOI:
10.1159/000448034. PMID:
27843813. PMCID:
PMC5091267.

117. Lefèvre CR, Peltier L, Damaj L, Valaize J, Bendavid C, Moreau C. 2020; Immunoassay disruption by high-dose biotin therapy: fair warning for neonatal care physicians. Pediatr Neurol. 112:8–9. DOI:
10.1016/j.pediatrneurol.2020.07.007. PMID:
32823140.

118. Avery G. 2019; Biotin interference in immunoassay: a review for the laboratory scientist. Ann Clin Biochem. 56:424–30. DOI:
10.1177/0004563219842231. PMID:
31023057.

119. Kummer S, Hermsen D, Distelmaier F. 2016; Biotin treatment mimicking Graves' disease. N Engl J Med. 375:704–6. DOI:
10.1056/NEJMc1602096. PMID:
27783908.

120. Piketty ML, Prie D, Sedel F, Bernard D, Hercend C, Chanson P, et al. 2017; High-dose biotin therapy leading to false biochemical endocrine profiles: validation of a simple method to overcome biotin interference. Clin Chem Lab Med. 55:817–25. DOI:
10.1515/cclm-2016-1183. PMID:
28222020.

121. Biscolla RPM, Chiamolera MI, Kanashiro I, Maciel RMB, Vieira JGH. 2017; A single 10 mg oral dose of biotin interferes with thyroid function tests. Thyroid. 27:1099–100. DOI:
10.1089/thy.2016.0623. PMID:
28614993.
123. Grimsey P, Frey N, Bendig G, Zitzler J, Lorenz O, Kasapic D, et al. 2017; Population pharmacokinetics of exogenous biotin and the relationship between biotin serum levels and
in vitro immunoassay interference. Int J Pharmacokinet. 2:247–56. DOI:
10.4155/ipk-2017-0013.
124. Peyro Saint Paul L, Debruyne D, Bernard D, Mock DM, Defer GL. 2016; Pharmacokinetics and pharmacodynamics of MD1003 (high-dose biotin) in the treatment of progressive multiple sclerosis. Expert Opin Drug Metab Toxicol. 12:327–44. DOI:
10.1517/17425255.2016.1136288. PMID:
26699811.

125. Bowen R, Benavides R, Colón-Franco JM, Katzman BM, Muthukumar A, Sadrzadeh H, et al. 2019; Best practices in mitigating the risk of biotin interference with laboratory testing. Clin Biochem. 74:1–11. DOI:
10.1016/j.clinbiochem.2019.08.012. PMID:
31473202.

126. Gifford JL, de Koning L, Sadrzadeh SMH. 2019; Strategies for mitigating risk posed by biotin interference on clinical immunoassays. Clin Biochem. 65:61–3. DOI:
10.1016/j.clinbiochem.2018.12.007. PMID:
30582902.

127. Mzougui S, Favresse J, Soleimani R, Fillée C, Gruson D. 2020; Biotin interference: evaluation of a new generation of electrochemiluminescent immunoassays for high-sensitive troponin T and thyroid-stimulating hormone testing. Clin Chem Lab Med. 58:2037–45. DOI:
10.1515/cclm-2020-0214. PMID:
32324155.

128. Lam L, Kyle CV. 2017; A simple method to detect biotin interference on immunoassays. Clin Chem Lab Med. 55:e104–6. DOI:
10.1515/cclm-2017-0059. PMID:
28306526.

129. Trambas C, Lu Z, Yen T, Sikaris K. 2018; Depletion of biotin using streptavidin-coated microparticles: a validated solution to the problem of biotin interference in streptavidin-biotin immunoassays. Ann Clin Biochem. 55:216–26. DOI:
10.1177/0004563217707783. PMID:
28406314.

131. Rulander NJ, Cardamone D, Senior M, Snyder PJ, Master SR. 2013; Interference from anti-streptavidin antibody. Arch Pathol Lab Med. 137:1141–6. DOI:
10.5858/arpa.2012-0270-CR. PMID:
23899071.

132. Verougstraete N, Berth M, Vaneechoutte M, Delanghe J, Callewaert N. 2020; Interference of anti-streptavidin antibodies in immunoassays: A very rare phenomenon or a more common finding? Clin Chem Lab Med. 58:1673–80. DOI:
10.1515/cclm-2019-1064. PMID:
31782946.

133. Dahll LK, Haave EM, Dahl SR, Aas FE, Thorsby PM. 2021; Endogenous anti-streptavidin antibodies causing erroneous laboratory results more common than anticipated. Scand J Clin Lab Invest. 81:92–103. DOI:
10.1080/00365513.2020.1858493. PMID:
33502256.

134. Berth M, Willaert S, De Ridder C. 2018; Anti-streptavidin IgG antibody interference in anti-cyclic citrullinated peptide (CCP) IgG antibody assays is a rare but important cause of false-positive anti-CCP results. Clin Chem Lab Med. 56:1263–8. DOI:
10.1515/cclm-2017-1153. PMID:
29466233.

135. Raverot V, Bordeau É, Periot C, Perrin P, Chardon L, Plotton I, et al. 2019; Letter to the editor: A case of laboratory-generated "thyroid dysfunction". Ann Endocrinol (Paris). 80:140–1. DOI:
10.1016/j.ando.2018.10.001. PMID:
30685059.

136. Lam L, Bagg W, Smith G, Chiu WW, Middleditch MJ, Lim JC, et al. 2018; Apparent hyperthyroidism caused by biotin-like interference from IgM anti-streptavidin antibodies. Thyroid. 28:1063–7. DOI:
10.1089/thy.2017.0673. PMID:
29808739.

137. Peltier L, Massart C, Moineau MP, Delhostal A, Roudaut N. 2016; Anti-streptavidin interferences in Roche thyroid immunoassays: a case report. Clin Chem Lab Med. 54:e11–4. DOI:
10.1515/cclm-2015-0350. PMID:
26154194.

138. Bayart JL, Favresse J, Melnik E, Lardinois B, Fillée C, Maiter D, et al. 2019; Erroneous thyroid and steroid hormones profile due to anti-streptavidin antibodies. Clin Chem Lab Med. 57:e255–8. DOI:
10.1515/cclm-2018-1355. PMID:
30903751.

139. Heijboer AC, Ijzerman RG, Bouman AA, Blankenstein MA. 2009; Two cases of antiruthenium antibody interference in Modular free thyroxine assay. Ann Clin Biochem. 46:263–4. DOI:
10.1258/acb.2009.008258. PMID:
19261677.

140. Buijs MM, Gorgels JP, Endert E. 2011; Interference by antiruthenium antibodies in the Roche thyroid-stimulating hormone assay. Ann Clin Biochem. 48:276–81. DOI:
10.1258/acb.2010.010160. PMID:
21441394.

141. Ando T, Yasui J, Inokuchi N, Usa T, Ashizawa K, Kamihara S, et al. 2007; Non-specific activities against ruthenium crosslinker as a new cause of assay interference in an electrochemilluminescent immunoassay. Intern Med. 46:1225–9. DOI:
10.2169/internalmedicine.46.0188. PMID:
17675774.

142. Ohba K, Noh JY, Unno T, Satoh T, Iwahara K, Matsushita A, et al. 2012; Falsely elevated thyroid hormone levels caused by anti-ruthenium interference in the Elecsys assay resembling the syndrome of inappropriate secretion of thyrotropin. Endocr J. 59:663–7. DOI:
10.1507/endocrj.EJ12-0089. PMID:
22673200.

143. Zaninotto M, Tognon C, Venturini R, Betterle C, Plebani M. 2014; Interference in thyroid hormones with Roche immunoassays: an unfinished story. Clin Chem Lab Med. 52:e269–70. DOI:
10.1515/cclm-2014-0454. PMID:
24960154.

144. Lim YY, Ong L, Loh TP, Sethi SK, Sng AAJ, Loke KY, et al. 2018; A diagnostic curiosity of isolated androstenedione elevation due to autoantibodies against horseradish peroxidase label of the immunoassay. Clin Chim Acta. 476:103–6. DOI:
10.1016/j.cca.2017.11.025. PMID:
29175172.

145. Maharjan AS, Wyness SP, Ray JA, Willcox TL, Seiter JD, Genzen JR. 2019; Detection and characterization of estradiol (E2) and unconjugated estriol (uE3) immunoassay interference due to anti-bovine alkaline phosphatase (ALP) antibodies. Pract Lab Med. 17:e00131. DOI:
10.1016/j.plabm.2019.e00131. PMID:
31538105. PMCID:
PMC6745434.

146. Sofronescu AG, Ross M, Rush E, Goldner W. 2018; Spurious testosterone laboratory results in a patient taking synthetic alkaline phosphatase (asfotase alfa). Clin Biochem. 58:118–21. DOI:
10.1016/j.clinbiochem.2018.04.024. PMID:
29709501.

148. Altawallbeh G, Karger AB. 2019; Letter to the Editor: assay-specific spurious ACTH results lead to misdiagnosis, unnecessary testing, and surgical misadventure-A case series. J Endocr Soc. 4:bvz011. DOI:
10.1210/jendso/bvz011. PMID:
32104751. PMCID:
PMC7035212.

149. Luzzi VI, Scott MG, Gronowski AM. 2003; Negative thyrotropin assay interference associated with an IgGkappa paraprotein. Clin Chem. 49:709–10. DOI:
10.1373/49.4.709. PMID:
12651842.
150. Imperiali M, Jelmini P, Ferraro B, Keller F, della Bruna R, Balerna M, et al. 2010; Interference in thyroid-stimulating hormone determination. Eur J Clin Invest. 40:756–8. DOI:
10.1111/j.1365-2362.2010.02315.x. PMID:
20546017.

151. Covinsky M, Laterza O, Pfeifer JD, Farkas-Szallasi T, Scott MG. 2000; An IgM lambda antibody to Escherichia coli produces false-positive results in multiple immunometric assays. Clin Chem. 46:1157–61. DOI:
10.1093/clinchem/46.8.1157. PMID:
10926897.
152. Whittle E, de Waal E, Huynh T, Treacy O, Morton A. 2021; Pre-analytical mysteries: A case of severe hypervitaminosis D and mild hypercalcaemia. Biochem Med (Zagreb). 31:011001. DOI:
10.11613/BM.2021.011001. PMID:
33380896. PMCID:
PMC7745154.

153. Ismail AA. 2009; Interference from endogenous antibodies in automated immunoassays: what laboratorians need to know. J Clin Pathol. 62:673–8. DOI:
10.1136/jcp.2008.055848. PMID:
19638536.

154. Hager HB, Bolstad N, Warren DJ, Ness MV, Seierstad B, Lindberg M. 2021; Falsely markedly elevated 25-hydroxyvitamin D in patients with monoclonal gammopathies. Clin Chem Lab Med. 59:663–9. DOI:
10.1515/cclm-2020-1411. PMID:
33119540.

155. Ong MW, Salota R, Reeman T, Lapsley M, Jones L. 2017; Artefactual 25-OH vitamin D concentration in multiple myeloma. Ann Clin Biochem. 54:716–20. DOI:
10.1177/0004563217690175. PMID:
28068803.

156. Visser WE, Peeters RP. 2020; Interpretation of thyroid function tests during pregnancy. Best Pract Res Clin Endocrinol Metab. 34:101431. DOI:
10.1016/j.beem.2020.101431. PMID:
32863110.

157. Khoo S, Lyons G, McGowan A, Gurnell M, Oddy S, Visser WE, et al. 2020; Familial dysalbuminaemic hyperthyroxinaemia interferes with current free thyroid hormone immunoassay methods. Eur J Endocrinol. 182:533–8. DOI:
10.1530/EJE-19-1021. PMID:
32213658. PMCID:
PMC7222281.

158. Cartwright D, O'Shea P, Rajanayagam O, Agostini M, Barker P, Moran C, et al. 2009; Familial dysalbuminemic hyperthyroxinemia: A persistent diagnostic challenge. Clin Chem. 55:1044–6. DOI:
10.1373/clinchem.2008.120303. PMID:
19282355.

159. Ross HA, de Rijke YB, Sweep FC. 2011; Spuriously high free thyroxine values in familial dysalbuminemic hyperthyroxinemia. Clin Chem. 57:524–5. DOI:
10.1373/clinchem.2010.158170. PMID:
21149501.

160. Kragh-Hansen U, Galliano M, Minchiotti L. 2017; Clinical, genetic, and protein structural aspects of familial dysalbuminemic hyperthyroxinemia and hypertriiodothyroninemia. Front Endocrinol (Lausanne). 8:297. DOI:
10.3389/fendo.2017.00297. PMID:
29163366. PMCID:
PMC5671950.

161. DeCosimo DR, Fang SL, Braverman LE. 1987; Prevalence of familial dysalbuminemic hyperthyroxinemia in Hispanics. Ann Intern Med. 107:780–1. DOI:
10.7326/0003-4819-107-5-780_2. PMID:
3662294.

163. Campi I, Covelli D, Moran C, Fugazzola L, Cacciatore C, Orlandi F, et al. 2020; The differential diagnosis of discrepant thyroid function tests: insistent pitfalls and updated flow-chart based on a long-standing experience. Front Endocrinol (Lausanne). 11:432. DOI:
10.3389/fendo.2020.00432. PMID:
32733382. PMCID:
PMC7358450.

164. Dieu X, Bouzamondo N, Briet C, Illouz F, Moal V, Boux de Casson F, et al. 2020; Familial dysalbuminemic hyperthyroxinemia: an underdiagnosed entity. J Clin Med. 9:2105. DOI:
10.3390/jcm9072105. PMID:
32635414. PMCID:
PMC7408830.

165. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. 2007; Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 92:405–13. DOI:
10.1210/jc.2006-1864. PMID:
17090633.
167. Faye SA, Groome N, Masica R, Kertez G. 2014; Investigation and resolution of the effect of an interfering factor in the Beckman Coulter anti-Müllerian hormone (AMH) Gen II ELISA assay. Hum Reprod. 29:331.
168. Turner KA, Larson BJ, Kreofsky NC, Willrich MAV, Bornhorst JA, Algeciras-Schimnich A. 2020; Assessment of complement interference in anti-Müllerian hormone immunoassays. Clin Chem Lab Med. 58:e8–10. DOI:
10.1515/cclm-2019-0496. PMID:
31815379.

169. Monaghan PJ, Kyriacou A, Sturgeon C, Davies A, Trainer PJ, White A, et al. 2016; Proopiomelanocortin interference in the measurement of adrenocorticotrophic hormone: a United Kingdom National External Quality Assessment Service study. Clin Endocrinol (Oxf). 85:569–74. DOI:
10.1111/cen.13118. PMID:
27256168.

170. Lauro C, Corcuff J-B, Brossaud J, Georges A. 2020; What to do in the event of a suspicion of analytical interference during an immunoassay? Ann Biol Clin (Paris). 78:70–3. DOI:
10.1684/abc.2020.1514. PMID:
32108582.

171. Gurnell M, Halsall DJ, Chatterjee VK. 2011; What should be done when thyroid function tests do not make sense? Clin Endocrinol (Oxf). 74:673–8. DOI:
10.1111/j.1365-2265.2011.04023.x.

172. Cavalier E, Carlisi A, Chapelle JP, Delanaye P. 2008; False positive PTH results: an easy strategy to test and detect analytical interferences in routine practice. Clin Chim Acta. 387:150–2. DOI:
10.1016/j.cca.2007.08.019. PMID:
17904113.

173. Ellis MJ, Livesey JH. 2005; Techniques for identifying heterophile antibody interference are assay specific: study of seven analytes on two automated immunoassay analyzers. Clin Chem. 51:639–41. DOI:
10.1373/clinchem.2004.043869. PMID:
15650033.

174. Choy KW, Teng J, Wijeratne N, Tan CY, Doery JC. 2017; Immunoassay interference complicating management of Cushing's disease: the onus is on the clinician and the laboratory. Ann Clin Biochem. 54:183–4. DOI:
10.1177/0004563216657362. PMID:
27303060.

175. Faix JD. 2013; Principles and pitfalls of free hormone measurements. Best Pract Res Clin Endocrinol Metab. 27:631–45. DOI:
10.1016/j.beem.2013.06.007. PMID:
24094635.

176. Ismail AA. 2007; On detecting interference from endogenous antibodies in immunoassays by doubling dilutions test. Clin Chem Lab Med. 45:851–4. DOI:
10.1515/CCLM.2007.152. PMID:
17617026.

177. Cole LA, Khanlian SA. 2004; Easy fix for clinical laboratories for the false-positive defect with the Abbott AxSym total β-hCG test. Clin Biochem. 37:344–9. DOI:
10.1016/j.clinbiochem.2004.03.001.

178. Santhana Krishnan SG, Pathalapati R, Kaplan L, Cobbs RK. 2006; Falsely raised TSH levels due to human anti‐mouse antibody interfering with thyrotropin assay. Postgrad Med J. 82:e27. DOI:
10.1136/pmj.2006.049809.
179. Interlaboratory survey of methods for measuring human anti-mouse antibodies. 1992; HAMA Survey Group. Clin Chem. 38:172–3. DOI:
10.1093/clinchem/38.1.172. PMID:
1733601.
180. Favresse J, Lardinois B, Nassogne MC, Preumont V, Maiter D, Gruson D. 2018; Anti-streptavidin antibodies mimicking heterophilic antibodies in thyroid function tests. Clin Chem Lab Med. 56:e160–3. DOI:
10.1515/cclm-2017-1027. PMID:
29447115.

181. Giovanella L, Giordani I, Imperiali M, Orlandi F, Trimboli P. 2018; Measuring procalcitonin to overcome heterophilic-antibody-induced spurious hypercalcitoninemia. Clin Chem Lab Med. 56:e191–3. DOI:
10.1515/cclm-2017-0993. PMID:
29257751.

182. Wong T, Shackleton CH, Covey TR, Ellis G. 1992; Identification of the steroids in neonatal plasma that interfere with 17 alpha-hydroxyprogesterone radioimmunoassays. Clin Chem. 38:1830–7. DOI:
10.1093/clinchem/38.9.1830. PMID:
1526021.

183. Makela SK, Ellis G. 1988; Nonspecificity of a direct 17 alpha-hydroxyprogesterone radioimmunoassay kit when used with samples from neonates. Clin Chem. 34:2070–5. DOI:
10.1093/clinchem/34.10.2070. PMID:
2971475.

184. Hawley JM, Owen LJ, Lockhart SJ, Monaghan PJ, Armston A, Chadwick CA, et al. 2016; Serum cortisol: an up-to-date assessment of routine assay performance. Clin Chem. 62:1220–9. DOI:
10.1373/clinchem.2016.255034. PMID:
27440512.

185. Vogeser M, Kratzsch J, Ju Bae Y, Bruegel M, Ceglarek U, Fiers T, et al. 2017; Multicenter performance evaluation of a second generation cortisol assay. Clin Chem Lab Med. 55:826–35. DOI:
10.1515/cclm-2016-0400. PMID:
27898397.

186. Thomas CM, van den Berg RJ, Segers MF, Bartelink ML, Thien T. 1993; Inaccurate measurement of 17 beta-estradiol in serum of female volunteers after oral administration of milligram amounts of micronized 17 beta-estradiol. Clin Chem. 39:2341–2. DOI:
10.1093/clinchem/39.11.2341. PMID:
8222233.

187. Nahoul K, Dehennin L, Scholler R. 1987; Radioimmunoassay of plasma progesterone after oral administration of micronized progesterone. J Steroid Biochem. 26:241–9. DOI:
10.1016/0022-4731(87)90078-1. PMID:
3560939.

188. Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, et al. 2003; Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 49:1381–95. DOI:
10.1373/49.8.1381. PMID:
12881456.

189. Warner MH, Kane JW, Atkin SL, Kilpatrick ES. 2006; Dehydroepiandrosterone sulphate interferes with the Abbott Architect direct immunoassay for testosterone. Ann Clin Biochem. 43:196–9. DOI:
10.1258/000456306776865034. PMID:
16704754.

190. Piketty ML, d'Herbomez M, Le Guillouzic D, Lebtahi R, Cosson E, Dumont A, et al. 1996; Clinical comparison of three labeled-antibody immunoassays of free triiodothyronine. Clin Chem. 42:933–41. DOI:
10.1093/clinchem/42.6.933. PMID:
8665686.

191. Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S. 2014; Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol. 14:33. DOI:
10.1186/1472-6890-14-33. PMID:
25071417. PMCID:
PMC4112981.

192. Dias ML, Vieira JG, Abucham J. 2013; Detecting and solving the interference of pregnancy serum, in a GH immunometric assay. Growth Horm IGF Res. 23:13–8. DOI:
10.1016/j.ghir.2012.11.001. PMID:
23206731.

193. Schiettecatte J, Strasser O, Anckaert E, Smitz J. 2016; Performance evaluation of an automated electrochemiluminescent calcitonin (CT) immunoassay in diagnosis of medullary thyroid carcinoma. Clin Biochem. 49:929–31. DOI:
10.1016/j.clinbiochem.2016.05.006. PMID:
27182955.

194. Hillebrand JJ, Siegelaar SE, Heijboer AC. 2020; Falsely decreased thyroglobulin levels in a patient with differentiated thyroid carcinoma. Clin Chim Acta. 509:217–9. DOI:
10.1016/j.cca.2020.06.027. PMID:
32561346.

195. Cormano J, Mackay G, Holschneider C. 2015; Gestational trophoblastic disease diagnosis delayed by the hook effect. Obstet Gynecol. 126:811–4. DOI:
10.1097/AOG.0000000000000860. PMID:
26132453.

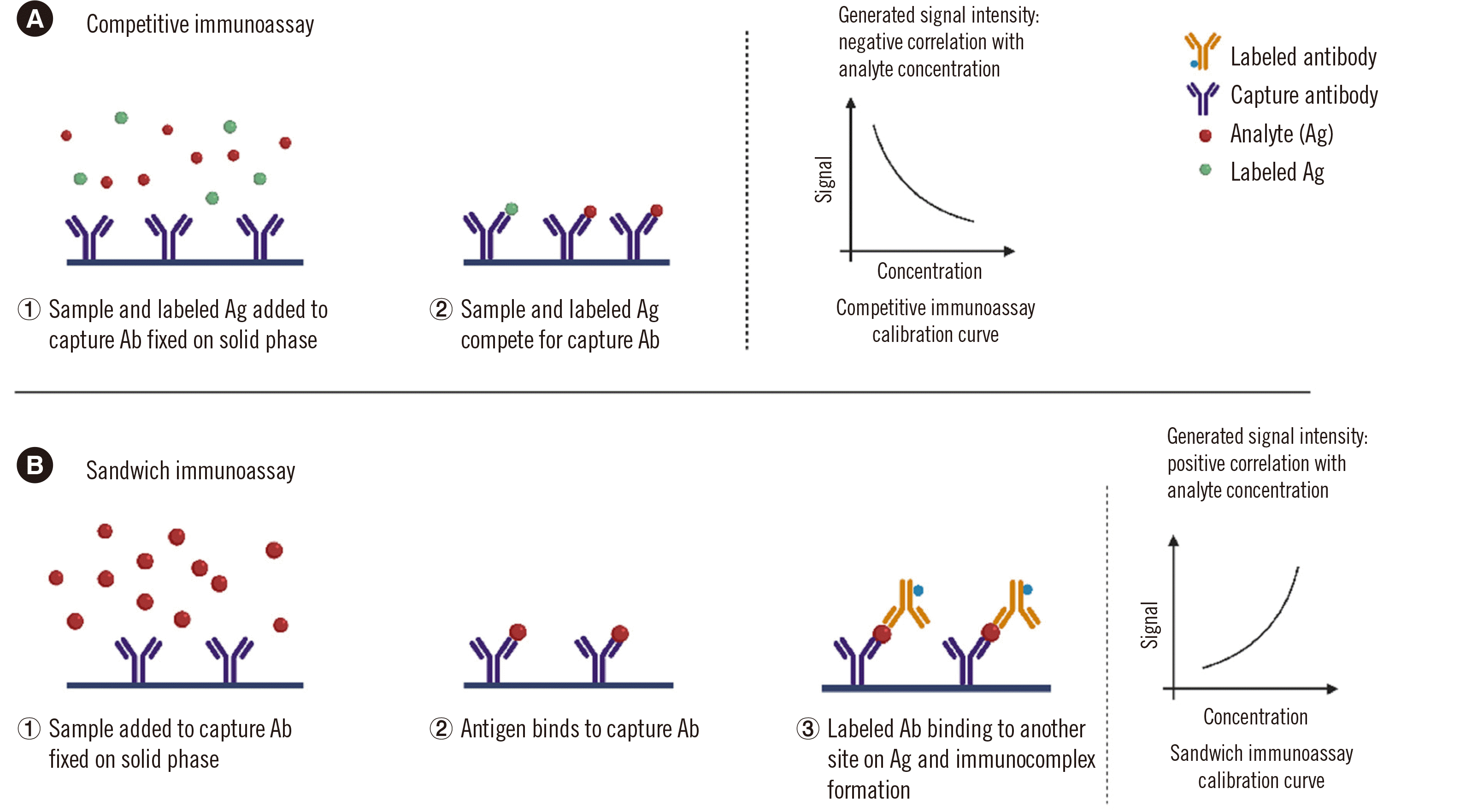
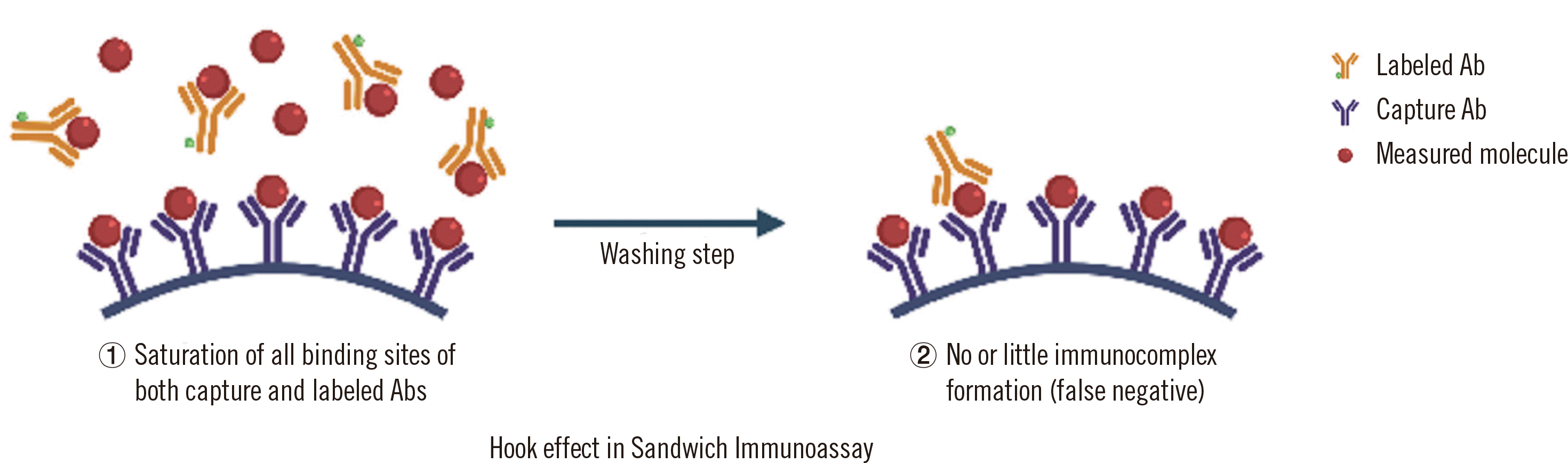
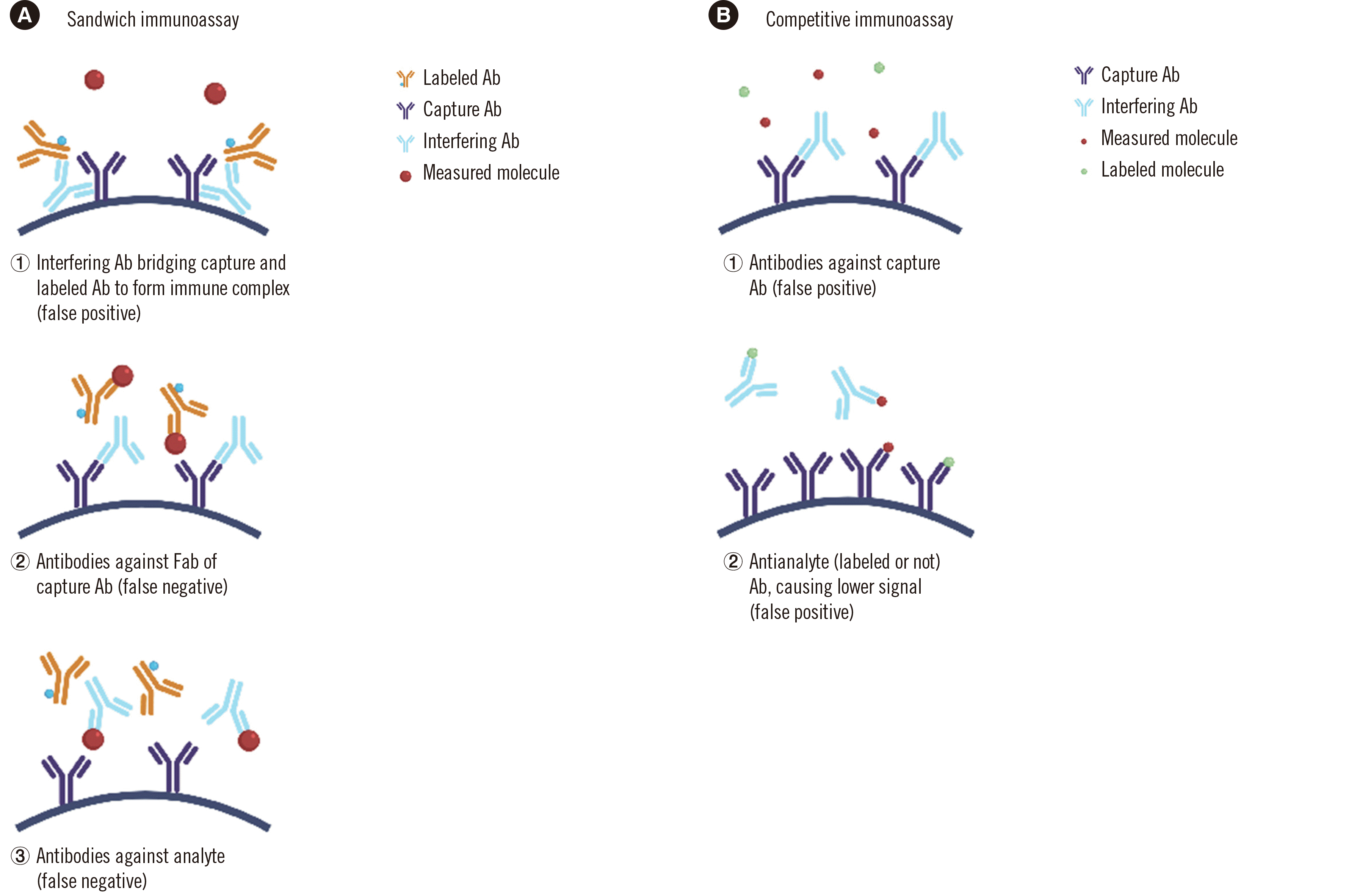




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download