This article has been
cited by other articles in ScienceCentral.
Abstract
Since February 26, 2021, when vaccination against coronavirus disease 2019 (COVID-19) began in South Korea, patients who visited the Korea University Guro Hospital with suspected adverse events after COVID-19 vaccination were monitored actively with interest. We encountered five unusual cases of polyarthralgia and myalgia syndrome in patients who received the ChAdOx1 nCOV-19 (AstraZeneca) vaccine. The patients (median age 67 years) were not previously diagnosed with arthropathy and rheumatologic diseases. They developed fever, myalgia, joint pain, and swelling three to seven days after vaccination. The symptoms persisted for up to 47 days despite antipyretic treatment. Arthralgia occurred in multiple joints, including small and large joints. A whole-body Technetium-99m methylene diphosphonate bone scan revealed unusual uptakes in the affected joints. Non-steroidal anti-inflammatory drugs with or without prednisolone relieved the symptoms of all patients. Further monitoring is required to clarify the long-term prognosis of this syndrome.
Go to :

Graphical Abstract
Go to :

Keywords: COVID-19, Vaccine, Safety, Adverse Event, Arthritis
As a mitigation strategy against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, coronavirus disease 2019 (COVID-19) vaccination started in South Korea on February 26, 2021. Approximately 22 million doses of the coronavirus disease 2019 vaccines have been administered in South Korea as of 15 July 2021. Among these were about 12 million doses of the ChAdOx1 nCOV-19 (AstraZeneca) vaccine.
1 The ChAdOx1 nCOV-19 vaccine has been primarily administered to healthcare workers and individuals aged 65–74 years old. An early safety survey showed that ChAdOx1 nCOV-19 vaccine recipients frequently experienced fever (38.7% of survey participants), myalgia (80.8%), and arthralgia (48.7%). However, most symptoms resolved within two days after vaccination.
2 We report five unusual cases of polyarthralgia and myalgia syndrome in ChAdOx1 nCOV-19 vaccine recipients. The patients were asymptomatic during the first one to two days after the administration of the first dose. However, they developed fever, myalgia, joint pain, and swelling, three to seven days after the first dose. These symptoms persisted for more than 10 days despite antipyretic treatment.
From February 26, 2021 to July 15, 2021, patients who visited the Korea University Guro Hospital (a tertiary referral hospital) with suspected adverse events after COVID-19 vaccination were monitored actively with interest. During that period, five patients presented with persistent polyarthralgia and myalgia that appeared similar to rheumatologic diseases. All patients showed no evidence of SARS-CoV-2 infection on real-time reverse transcription-polymerase chain reaction (Allplex™ SARS-CoV-2 Assay Seegene) assay. The clinical characteristics of these patients are presented in
Table 1. All patients were female (median age 67 years) without previously diagnosed arthropathy and rheumatologic diseases. Arthralgia occurred in both small and large joints (patient 1 had arthralgia in both hands and feet; patient 2 had arthralgia in both hands and ankles; patient 3 had arthralgia in both hands, feet, and shoulders; patient 4 had arthralgia in both hands and legs; and patient 5 had arthralgia in the neck, back, both elbows, knees, hands, and feet). On physical examination, the patients' vital signs were stable, with no erythema in the joint area; however, generalized edema of the small joint was observed. Laboratory tests revealed elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), D-dimer (DD) and lactate dehydrogenase (LDH) levels. The creatine phosphokinase and rheumatoid factor levels were normal (mean values, ESR 70 mm/h; CRP 100 mg/L; DD 2.55 µg/mL fibrinogen-equivalent units; LDH 620 IU/L). The platelet counts and prothrombin times were normal in four patients (patient 1–4). The prothrombin time of patient 5 was mildly prolonged (78%), but thrombosis was not noted on the computed tomography scan. In two patients (1 and 2,
Table 1), the anti-nuclear and anti-neutrophil cytoplasmic antibody tests were slightly positive, while the extractable nuclear antigen panel and myeloperoxidase/proteinase-3 antibodies were negative. The anti-cyclic citrullinated peptide antibody test was performed for four patients (patients 1–3, and 5) with negative results. A whole-body Technetium-99m methylene diphosphonate bone scan revealed unusual uptakes in the affected joints (
Fig. 1). Non-steroidal anti-inflammatory drugs with or without prednisolone relieved the symptoms of all patients. Patient 4 developed similar symptoms after the administration of the second vaccine dose.
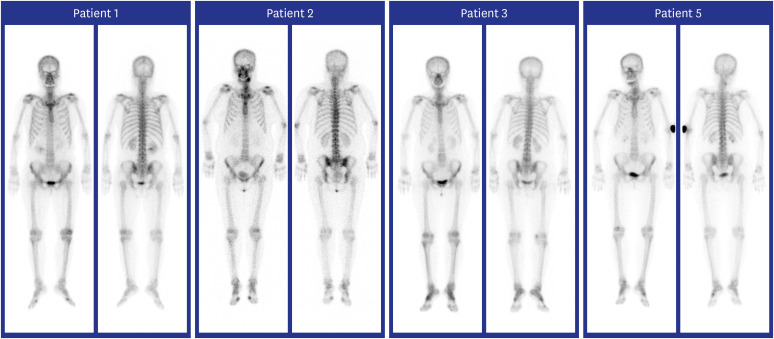 | Fig. 1
Whole-body bone scan findings of patients with polyarthralgia and myalgia syndrome in ChAdOx1 nCOV-19 recipients. Increased uptakes in both first MTP and left knee joints were seen in patient 1; increased uptakes in both shoulders, elbows, wrists, ankles, multiple fingers, first MTP, and left knee joints in patient 2; increased uptakes in both wrists, ankles, multiple fingers, first MTP, and right knee joints in patient 3; increased uptakes in the cervical spine, both wrists, hands, fingers, and left foot joints in patient 5.
MTP = metatarsophalangeal.

|
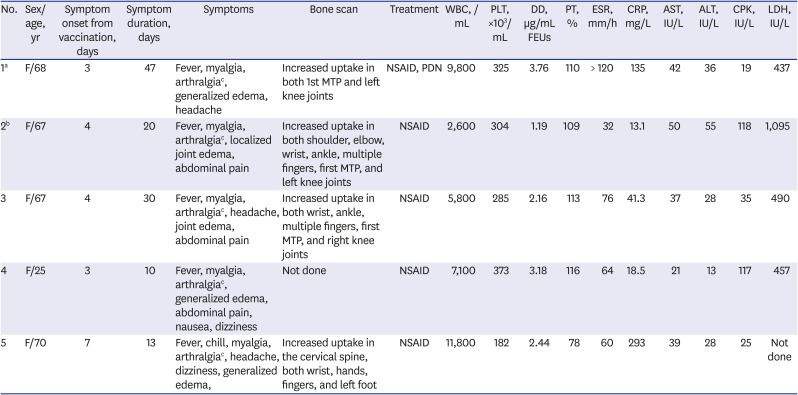
Table 1
Characteristics of patients with polyarthralgia and myalgia syndrome after the administration of ChAdOx1 nCOV-19
|
No. |
Sex/age, yr |
Symptom onset from vaccination, days |
Symptom duration, days |
Symptoms |
Bone scan |
Treatment |
WBC, /mL |
PLT, ×103/mL |
DD, µg/mL FEUs |
PT, % |
ESR, mm/h |
CRP, mg/L |
AST, IU/L |
ALT, IU/L |
CPK, IU/L |
LDH, IU/L |
|
1a
|
F/68 |
3 |
47 |
Fever, myalgia, arthralgiac, generalized edema, headache |
Increased uptake in both 1st MTP and left knee joints |
NSAID, PDN |
9,800 |
325 |
3.76 |
110 |
> 120 |
135 |
42 |
36 |
19 |
437 |
|
2b
|
F/67 |
4 |
20 |
Fever, myalgia, arthralgiac, localized joint edema, abdominal pain |
Increased uptake in both shoulder, elbow, wrist, ankle, multiple fingers, first MTP, and left knee joints |
NSAID |
2,600 |
304 |
1.19 |
109 |
32 |
13.1 |
50 |
55 |
118 |
1,095 |
|
3 |
F/67 |
4 |
30 |
Fever, myalgia, arthralgiac, headache, joint edema, abdominal pain |
Increased uptake in both wrist, ankle, multiple fingers, first MTP, and right knee joints |
NSAID |
5,800 |
285 |
2.16 |
113 |
76 |
41.3 |
37 |
28 |
35 |
490 |
|
4 |
F/25 |
3 |
10 |
Fever, myalgia, arthralgiac, generalized edema, abdominal pain, nausea, dizziness |
Not done |
NSAID |
7,100 |
373 |
3.18 |
116 |
64 |
18.5 |
21 |
13 |
117 |
457 |
|
5 |
F/70 |
7 |
13 |
Fever, chill, myalgia, arthralgiac, headache, dizziness, generalized edema, |
Increased uptake in the cervical spine, both wrist, hands, fingers, and left foot |
NSAID |
11,800 |
182 |
2.44 |
78 |
60 |
293 |
39 |
28 |
25 |
Not done |

In this report, we presented five cases of polyarthralgia and myalgia syndrome that were suspected to be adverse events after COVID-19 vaccination. This syndrome presented acutely and persisted for up to 47 days. Clinically, it mimicked polymyalgia rheumatica, rheumatoid arthritis, or other systemic inflammatory arthritis. Further monitoring is required to clarify the long-term prognosis of this syndrome. Based on the acute onset, laboratory findings, and clinical response to medications, the adenovirus vector-induced cytokine activation (rather than autoantibody formation) was likely involved in the main pathogenesis. In the mouse model, systemic administration of adenoviral vectors triggered the innate immune system to elicit an acute inflammatory response, characterized by inflammatory cytokine secretion (tumor necrosis factor-α, interleukin [IL]-6, IL-12, etc.) and tissue injury.
345 The effect of the adenoviral vectors on the organs and immune response might be variable depending on the patient's age. Cerebral venous sinus thrombosis, caused by platelet factor 4-heparin antibodies, more frequently affected young adults, who received the ChAdOx-1 nCOV-19 vaccine, than adults aged over 50 years.
678 In addition, the younger recipients of the ChAdOx-1 nCOV-19 vaccine frequently complained of fever and muscle pain, which were likely due to proinflammatory cytokine activation.
2 These symptoms typically resolved within 48 hours. However, although the reason of age-dependent difference is unclear, cytokine activation may induce persistent systemic inflammatory arthritis in some elderly people. More studies are needed to clarify the pathogenesis further. The occurrence of this acute syndrome should also be investigated in the human adenovirus vectored vaccines.
Although data on COVID-19 vaccine are scarce, rheumatologic manifestation after vaccination has been previously reported.
910 Thus, it is unclear whether COVID-19 vaccine is more likely to cause rheumatologic manifestations than other vaccines. These manifestations may also occur in SARS-CoV-2 infection; considering the hyperinflammatory response of COVID-19, COVID-19 itself could be a risk factor for inflammatory musculoskeletal diseases.
11121314 With the information available to date, the risk for this adverse event does not outweigh the benefits of COVID-19 vaccination.
15 However, we could find several cases with similar musculoskeletal symptoms in a relatively short time period of time through a hospital-based active surveillance system. Further studies are needed to determine whether cytokine activation was caused by adenoviral vector, SARS-CoV-2 spike protein, or other vaccine components. It is necessary to carefully monitor whether the number of cases of polyarthralgia and myalgia syndrome increases with COVID-19 vaccination through either multicenter active surveillance or healthcare big data analysis.
Ethics statement
This study was approved by the Institutional Review Board of Korea University Guro Hospital and the requirement for informed consent was waived (IRB No. 2021GR0415).
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download