This article has been
cited by other articles in ScienceCentral.
Abstract
Increasing rates of coronavirus disease 2019 (COVID-19) vaccination coverage will result in more vaccine-related side effects, including acute myocarditis. In Korea, we present a 24-year-old male with acute myocarditis following COVID-19 vaccination (BNT162b2). His chest pain developed the day after vaccination and cardiac biomarkers were elevated. Echocardiography showed minimal pericardial effusion but normal myocardial contractility. Electrocardiography revealed diffuse ST elevation in lead II, and V2-5. Cardiac magnetic resonance images showed the high signal intensity of T2- short tau inversion recovery image, the high value of T2 mapping sequence, and late gadolinium enhancement in basal inferior and inferolateral wall. It was presumed that COVID-19 mRNA vaccination was probably responsible for acute myocarditis. Clinical course of the patient was favorable and he was discharged without any adverse event.
Keywords: COVID-19, Myocarditis, Vaccination
INTRODUCTION
Coronavirus disease 2019 (COVID-19) has been globally disseminated and shows high infectivity and mortality.
1 In Korea, COVID-19 vaccination was initiated in March 2021, and as of now, approximately 14% of all Korean people received the second vaccination using one of four COVID vaccines available in Korea. However, with the increase of vaccination, the complications are also reported increasingly across the globe.
23
Among many complications, myalgia, febrile sensation, and mild fever are very common, but recently, growing numbers of acute myocarditis have been also reported following COVID-19 vaccination, particular in mRNA vaccine.
12345 Myocarditis is characterized with an inflammation of myocardium, which is also related with elevations of myocardial biomarkers, electrocardiographic changes, and chest pain or equivalent discomfort. Unfortunately, it is difficult to discriminate atypical chest pain of myocarditis from myalgia-like chest discomfort. However, knowledge of vaccination-related myocarditis would be important for physicians responsible for the ongoing herd immunity. Herein, we reported on a young male with acute myocarditis following COVID-19 mRNA vaccination.
CASE DESCRIPTION
A 24-year-old male presented to hospital with chest pain that was not related with effort or labor. The chest pain revealed a duration less than 2–3 minutes, an atypical dull nature on the substernal area, and non-radiating and constant discomfort. This chest pain was not relieved and persisted all day long for 2 days; therefore, he was transferred to our hospital from a local medical clinic.
One day before the chest pain, he had received his second dose of BNT162b2 (Pfizer-BioNTech COVID-19 vaccine). His first dose of COVID-19 vaccine was initiated 21 days prior, and he did not experience any discomfort or side effects of vaccination. Contrary to the first vaccination, on the day after the second dose, myalgia and fatigue developed without definite fever, but was tolerable and he could do his usual daily activity without discomfort. The next day, chest pain occurred and prompted him to visit our emergency department. With the respect to his personal history, he was healthy and did not smoke or drink alcohol. He denied any medication and did not have any underlying illness.
On presentation, his temperature was 36.4°C, blood pressure was 125/80 mmHg, heart rate was 85 beats per minute. Physical examination was unremarkable, and heart sounds were regular and without any murmur or friction. Regarding laboratory evaluation, cardiac biomarkers and inflammation makers were elevated: creatine kinase-MB fraction was high up to 15.3 ng/mL (normal: < 4.87 ng/mL), creatine phosphokinase 226 U/L (normal: < 190 U/L), troponin-I 2.28 ng/mL (normal: < 0.3 ng/mL), C-reactive protein 7.7 mg/dL (normal: < 0.5 mg/dL), erythrocyte sedimentation rate 27 mm/hour (normal: < 15 mm/hr). However, other blood test findings including N-terminal pro-B-natriuretic peptide level were within normal range, and severe acute respiratory syndrome coronavirus 2 with other serologic viral markers such as adenovirus, coxsackievirus B1, and parvovirus B19 were all negative.
Coronary computed tomographic angiography showed normal coronary arteries with no stenosis or occlusion. An electrocardiography showed the mild ST segment elevation in leads I, II, aVF, and V2-6 (
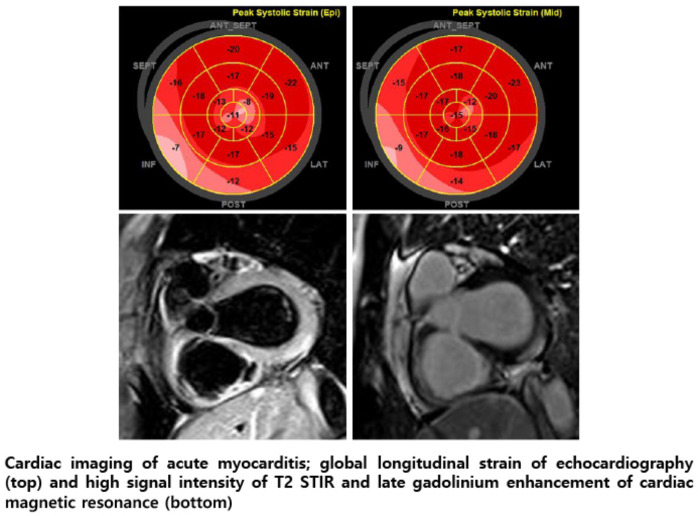
Fig. 1). Echocardiography showed normal myocardial contractility and minimal amount of pericardial effusion. 2D-based global longitudinal strain (GLS) of bull's map revealed the worsened strain value in basal inferior and inferolateral segments, particularly in epicardium than endocardium (
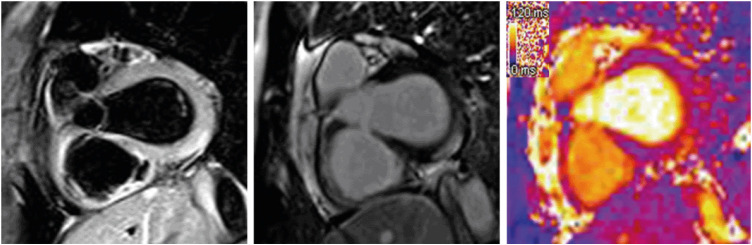
Fig. 2). Cardiac magnetic resonance (CMR) imaging (3 tesla, Magnetom VIDA, Siemens Healthineers, Germany) showed abnormal findings compatible with acute myocarditis according to revised 2018 Lake Louise criteria
6; high signal intensity on T2-weighted short tau inversion recovery images, elevated T2 value on T2 mapping sequence (51ms, normal reference value: 40ms), and sub-epicardial pattern of late gadolinium enhancement (LGE) in basal inferior and inferolateral segment (
Fig. 3). Based on these findings, acute myocarditis was finally diagnosed, and the patient was closely observed and treated with symptomatic therapy because of the minor episodes of chest discomfort. In recovery phase (5 days after admission), electrocardiographic ST segment started to recover in leads V2-6 (
Fig. 1), and GLS was fully recovered and cardiac biomarkers normalized (
Fig. 2).
Fig. 1
Dynamic ST segment changes. At emergency room, diffuse ST segment elevations are shown in leads I, II, aVF, and V2-6 (top). During recovery phase, ST segment elevations begin to be recovered from leads V2-6 (bottom).
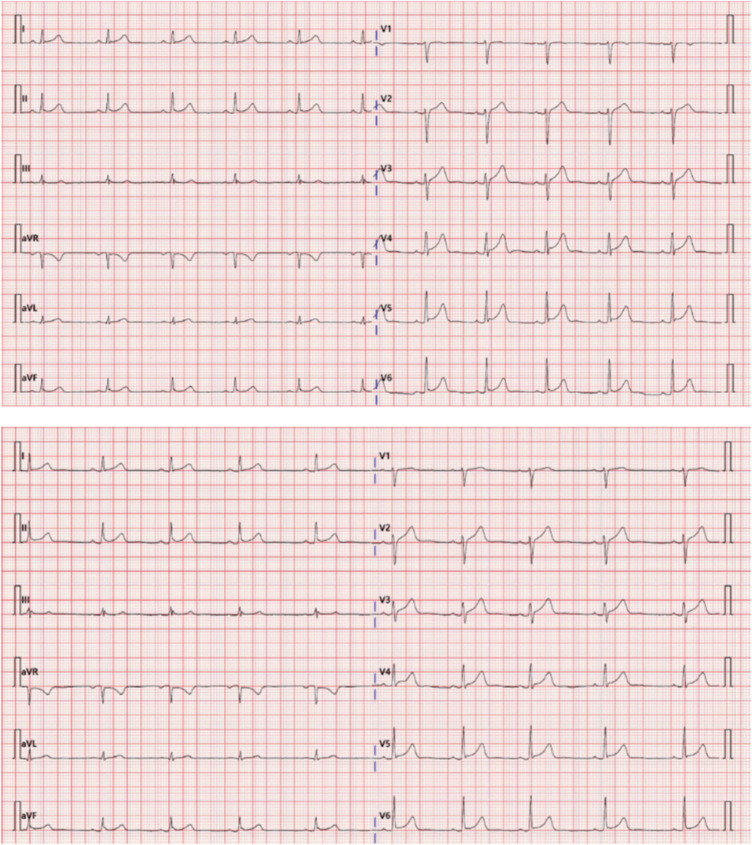
Fig. 2
Bull's eye map of GLS in echocardiography. At first day of admission, basal inferior wall shows reduced GLS severely from epicardium toward endocardium (top). However, at time of discharge, a reduced GLS was fully recovered (bottom).
GLS = global longitudinal strain.
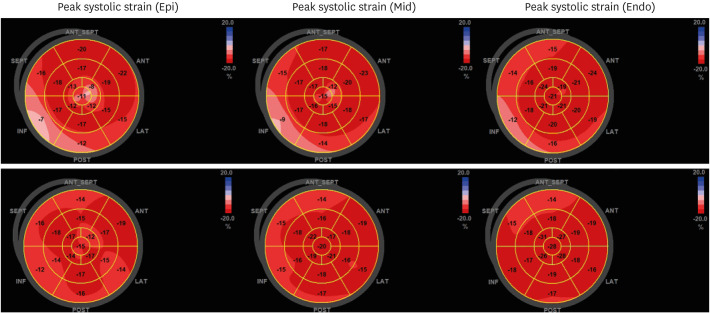
Fig. 3
Cardiac magnetic resonance imaging of acute myocarditis. Edema-sensitive T2-short tau inversion recovery shows high signal intensity on basal inferior-inferolateral segment (left). This region is compatible with late gadolinium enhancement (middle). Furthermore, T2 value is elevated up to 51 ms (reference of 40 ms) on same region on T2 map (right).
Ethics statement
This study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center (No. 2021-07-079) and the requirement for informed consent was obtained.
DISCUSSION
Until now, in Korea, four types of COVID-19 vaccinations are available and were initiated from late February in 2021: two mRNA-types by Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273), and another two vector-types by AstraZeneca (recombinant adenovirus type 26) and Janssen (recombinant adenovirus type 5). Because of the unprecedented COVID-19 infection, there are limited data about vaccine-related complications such as acute myocarditis.
The side effects or adverse reactions of vaccines are frequently related with its effectiveness and the response of the immune system. Despite these physiologic reactions, severe dangerous adverse events should be avoided. The review of literature indicated that the characteristics of patients with acute myocarditis were young males, particularly in their twenties or less than 30 years (up to 40 years) who were all vaccinated with the mRNA types.
7891011 Furthermore, those in the tenth were reported.
12 The occurrence of myocarditis was estimated to be 5-fold higher in patients under than over the twenties.
10 It is uncertain which vaccine between Pfizer-BioNTech and Moderna would be commonly related with acute myocarditis. Interestingly, only one case was reported following adenovirus-vector vaccine (Janssen).
9 Regarding the number of vaccinations, most myocarditis was related with a second dose of vaccination due to stronger immune response compared to the first dose, as shown in our case.
71013 The time onset of myocarditis from vaccination was less than 5 days.
14
Regarding the early detection or diagnosis of acute myocarditis, a strong suspicion should be required because it is difficult to distinguish chest discomfort between myocarditis and myalgia or flu-like symptoms. Otherwise, cardiac biomarkers and electrocardiography become the first aid to guide physicians for the appropriate reactions to myocarditis, like the surveillance of cardiac involvement with COVID-19 infection.
15 The role of cardiac imaging is useful to detect myocardial inflammation using echocardiography and CMR.
1416 Recently, 2D-based GLS can suggest the myocardial deformation, which worsens earlier than structural indicators, such as left ventricular ejection fraction.
17 As shown in our case, GLS worsening in epicardium was similar to the epicardial LGE of CMR which is regarded as a typical pattern of acute myocarditis. Therefore, given the feasibility of echocardiography, epicardial pattern of GLS worsening could be a screening test for acute myocarditis before CMR or cardiac biopsy.
Although severe serious acute myocarditis following COVID-19 vaccination has been rarely reported, rapid surveillance such as cardiac markers, electrocardiography, or cardiac imaging should be required for its early diagnosis in patients with chest discomfort within one week following vaccination.
ACKNOWLEDGMENTS
The authors would like to acknowledge J-H Jeon, RN and S-Y Kim, NR for significant contributions to this study. The present case report is published under agreement of the patient using his clinical data.
References
1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020; 324(8):782–793. PMID:
32648899.
2. Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, et al. Adverse events reported from COVID-19 vaccine trials: a systematic review. Indian J Clin Biochem. 2021; 27:1–13.

3. Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 2021; 9(5):467. PMID:
34066475.

4. Albert E, Aurigemma G, Saucedo J, Gerson DS. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021; 16(8):2142–2145. PMID:
34025885.

5. Deb A, Abdelmalek J, Iwuji K, Nugent K. Acute myocardial injury following COVID-19 vaccination: a case report and review of current evidence from vaccine adverse events reporting system database. J Prim Care Community Health. 2021; 12:21501327211029230. PMID:
34219532.

6. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018; 72(24):3158–3176. PMID:
30545455.
7. Abu Mouch S, Roguin A, Hellou E, Ishai A, Shoshan U, Mahamid L, et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021; 39(29):3790–3793. PMID:
34092429.

8. Albert E, Aurigemma G, Saucedo J, Gerson DS. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021; 16(8):2142–2145. PMID:
34025885.

9. Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. Forthcoming. 2021; DOI:
10.1161/CIRCULATIONAHA.121.055891.

11. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. Forthcoming. 2021; DOI:
10.1001/jamacardio.2021.2833.

12. Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. Forthcoming. 2021; DOI:
10.1542/peds.2021-052478.
13. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T
H1 T cell responses. Nature. 2020; 586(7830):594–599. PMID:
32998157.
14. Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. Forthcoming. 2021; DOI:
10.1001/jamacardio.2021.2828.

15. Kim IC, Song JE, Lee HJ, Park JH, Hyun M, Lee JY, et al. The implication of cardiac injury score on in-hospital mortality of coronavirus disease 2019. J Korean Med Sci. 2020; 35(39):e349. PMID:
33045772.

16. Matsubara D, Kauffman HL, Wang Y, Calderon-Anyosa R, Nadaraj S, Elias MD, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol. 2020; 76(17):1947–1961. PMID:
32890666.

17. Haji K, Marwick TH. Clinical utility of echocardiographic strain and strain rate measurements. Curr Cardiol Rep. 2021; 23(3):18. PMID:
33594493.








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download