DISCUSSION
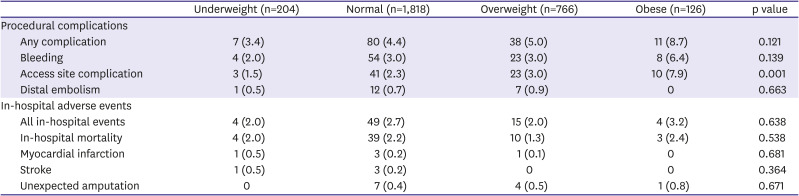
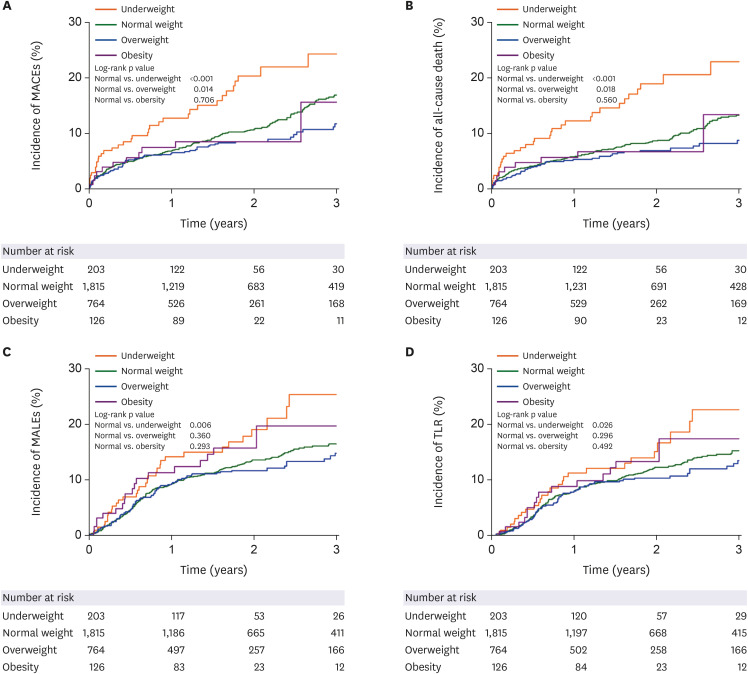
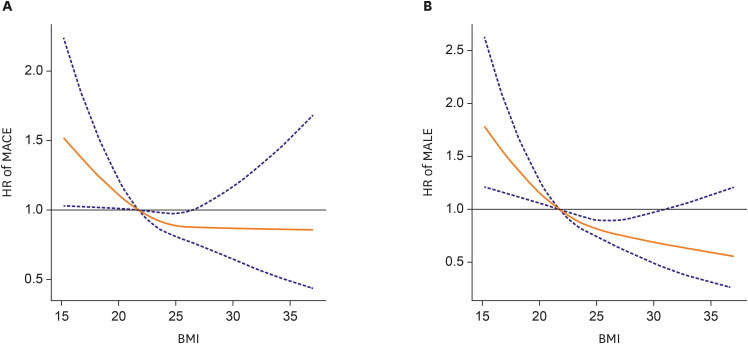
The main findings of our study are as follows: 1) In PAD patients, underweight was significantly associated with increased risk of MACE and MALE compared with normal weight. In addition, underweight also showed significantly increased risk for all-cause death, cardiac death, and TLR compared with normal weight. 2) Overweight was associated with lower risk of MACE, all-cause death, and cardiac death compared with normal weight. However, overweight showed no impact on MALE. 3) Obesity showed no significant association with MACE or MALE. 4) Underweight was identified as an independent predictor for MACE as well as for MALE. 5) There was a negative non-linear association between continuous BMI and the risk of MACE and MALE.
Our study findings are consistent with those of previous studies investigating the influence of BMI on prognosis for patients with PAD. Golledge et al.
5) reported an inverse association between BMI and the risk of death in patients with PAD. They found that underweight was associated with approximately two-fold higher mortality risk, while overweight and obesity were associated with about 30–40% decrease in mortality risk.
In another retrospective cohort study of CLI patients undergoing EVT for infrapopliteal artery lesions, the underweight group showed a higher death rate, whereas overweight and obese groups showed lower death rates than the normal weight group.
12) However, the weight status had no impact on MALE in that analysis.
A recent analysis using the National Inpatient Sample database showed that patients in the low BMI group were more likely to experience in-hospital death, MACE, open bypass surgery, or infection compared with the normal BMI group.
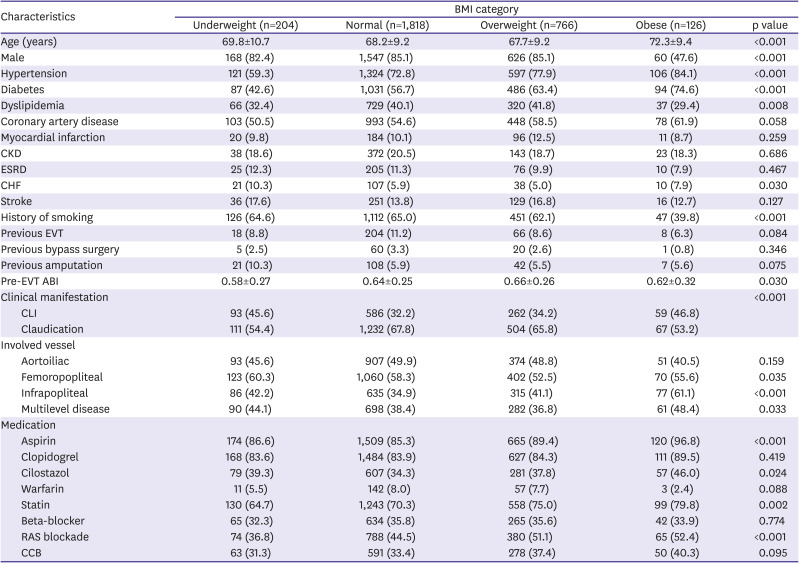
13) However, procedural complications, such as vascular complication or major bleeding, did not differ between the patient groups. In contrast, in the present study, we found no significant trend in in-hospital adverse events according to body weight status, possibly due to the small number of these events. However, incidences of access site complications were higher with increasing BMI. This may be explained by the greater depth of the access vessel, which may make it more difficult to precisely puncture the target vessel, and to apply effective hemostasis after the removal of vascular sheath.
14) Previous studies investigating the relationship between BMI and procedural complications have reported conflicting results, possibly due to differences in study populations and procedures.
15)16)
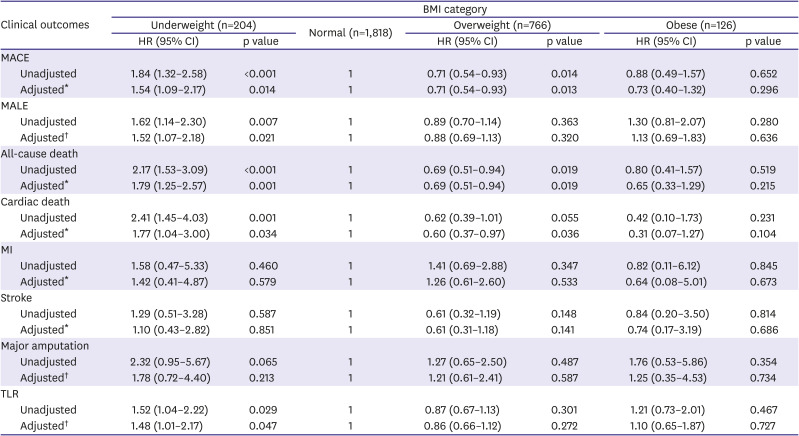
In the present study, underweight, end-stage renal disease, and CLI were identified as common independent predictors for MACE as well as for MALE. These predictors were also found in previous studies evaluating prognostic factors of PAD. Murata et al.
12) reported that underweight and chronic renal failure were independent predictors for death in patients with CLI after EVT for isolated infra-popliteal artery lesion. Kumakura et al.
17) revealed that low BMI, estimate glomerular filtration rate, and CLI were significant risk factors for both all-cause and cardiovascular death in PAD. However, to the best of our knowledge, there has been no study reporting underweight as an independent predictor of MALE in patients with PAD. In our study, underweight was an independent predictor not only for MACE, but also for MALE. Furthermore, the predictive power of underweight (HR, 1.510) for MALE was comparable with other traditional predictors such as CLI (HR, 1.704) and end-stage renal disease (HR, 2.063).
The mechanism of adverse effects of underweight on clinical outcomes of PAD is not well established. A possible explanation is that low BMI is associated with unfavorable clinical factors such as old age, malnutrition, or sarcopenia.
18)19)20) In our study, however, underweight remained a significant predictor for MACE and MALE even after adjustment for age and other covariates. Malnutrition, another plausible explanation for poor prognosis of underweight, is associated with sarcopenia, and causes loss of metabolic reserve. Malnutrition is frequently combined with inflammation in chronic diseases such as heart failure. Malnutrition, sarcopenia, and chronic inflammation aggravate one another, creating a vicious cycle of fragility, the so-called malnutrition-inflammation complex syndrome.
21)
Sarcopenia is frequently combined with PAD, and its prevalence increases with decreasing BMI.
22) In small single center study, sarcopenia has been shown to be associated with higher death rate in patients with CLI (HR, 3.22; 95% CI, 1.24–9.11; p=0.02).
23) The mechanism is not yet fully elucidated, however, aforementioned chronic inflammatory and protein wasting state of sarcopenia can be a plausible mechanism. Furthermore, level of adiponectin and carnitine, important anti-inflammory, anti-atherosclerotic, insulin sensitizing and pro-myogenic hormone, was reduced in sarcopenia.
24) It promotes metabolic dysfunction and atherosclerosis in sarcopenic patients, resulting in poor clinical prognosis. Recent national health data showed that preserved muscle mass plays a crucial role in better survival rate in same BMI category from underweight to obesity people.
25) It suggested sarcopenia could be more precise prognostic factor than BMI.
It is uncertain whether underweight results from an advanced atherosclerotic process of PAD or other comorbidities. Generally, physicians tend to consider underweight patients as having better lipid profiles and less CVD than higher body weight patients. This presumption may lead to CVD not being diagnosed in its early stage, and to underweight patients being managed less aggressively. In addition, the relatively low blood pressure in the underweight patients and their more sensitive reaction to low doses of drugs can present additional hurdles in prescribing medications.
A previous study demonstrated that good nutritional status is an independent predictor of better clinical outcomes in patients undergoing EVT.
26) However, there are currently no recommendations for underweight PAD patients to gain weight in order to improve clinical outcomes. Further studies are needed to elucidate the role of increasing muscle, nutritional support, and weight gain on prognosis of PAD in underweight patients.
Our study has some limitations. First, the present study is a retrospective analysis with potential selection and observational bias, and this type of study does not reveal cause-and-effect relationships. Baseline characteristics and medical treatment showed significant difference between the different patient groups. There were more diabetes and hypertension in the overweight and obesity group than in the underweight and in the normal weight group. Accordingly, the overweight and the obesity group required more antiplatelet, lipid lowering, and antihypertensive medications than the underweight or the normal weight group. We tried to adjust the analysis outcomes based on the baseline characteristics. Second, the study population was confined to patients with PAD undergoing EVT. Thus, the results cannot be generalized to all patients with PAD. Third, BMI was estimated just once upon hospitalization for the index procedure. Therefore, the impact of changes in body weight on clinical outcomes during follow-up was not taken into account. Lastly, because the present registry database did not include information regarding nutrition, muscle mass, or physical activity, more detailed analysis of clinical factors leading to the underweight state was not feasible.
In conclusion, in patients with PAD undergoing endovascular therapy, underweight is an independent predictor for MACE and MALE, whereas overweight was associated with lower risk of MACE than normal weight.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download