A 33-year-old man with no significant previous medical history received the first dose of the ChAdOx1 nCOV-19 vaccine (day 0). He reported mild symptoms in the evening of the day of vaccination (fever and headache). However, the symptoms improved after 2–3 days. On day 9 after vaccination, he developed a headache again and also experienced vomiting. He received treatment at a private clinic, but his symptoms did not improve and gradually worsened despite of symptomatic care. Twelve days after vaccination, he was admitted to the emergency room by ambulance due to the sudden onset of a tingling sensation in the right arm and mental change. He was alert when he left home, but became drowsy after arriving at the emergency department and developed neurological symptoms with dysarthria and right hemiparesis. He was afebrile, slightly hypertensive (155/90 mmHg), and the SARS-CoV-2 Xpert real-time reverse-transcriptase polymerase chain reaction assay of a nasopharyngeal and oropharyngeal swab was negative. The laboratory results are presented in
Table 1. The platelet count was 14,000/mm
3, C-Reactive Protein was 3.42 mg/L (reference value, < 5), fibrinogen was 77 mg/dL, and D-dimer was > 35.2 mg/L (reference value, < 0.5). There were no specific findings other than thrombocytopenia in the peripheral blood smear. Non-enhanced brain computed tomography identified a 5 × 4 × 3-cm
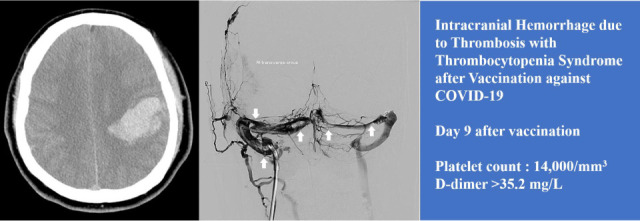
3-sized subcortical hematoma in the left parietal lobe and an adjacent subarachnoid hemorrhage, which suggested hemorrhagic venous infarction (
Fig. 1A). Two hours after arriving at the hospital, he was transferred to the neurosurgical department and admitted to the intensive care unit for further management. Three pint of Fresh Frozen Plasma and 8 pint of Platelet Concentrate were transfused immediately. Seven hours after arriving at the hospital, he became stuporous, and right hemiparesis became more severe from motor grade 2 to 1. On the magnetic resonance (MR) venogram, only the left sigmoid sinus and internal jugular vein were visualized, and the remaining dural venous sinus was not visualized, suggesting extensive CVT (
Fig. 1B). According to the Korean TTS guidelines, the patient was considered a probable case of TTS. We requested an enzyme-linked immunosorbent assay (ELISA) for antibodies against platelet factor 4 (PF4 ELISA Ab test) to confirm the diagnosis. Sixteen hours after arriving at the hospital, he received high-dose intravenous immunoglobulin (IVIG) 1,000 mg/kg daily and steroid (methylprednisolone 1.5 mg/kg/day). Heparin was not used upon arrival at the hospital and contraindicated. The symptoms did not improve even during IVIG and steroid maintenance therapy; thirty-six hours after arrival at the hospital, the level of consciousness deteriorated to semi-coma. Endovascular mechanical thrombectomy was performed for recanalization of the occluded dural venous sinus. Venography of the transverse sinus (
Fig. 1C) and superior sagittal sinus (
Fig. 1D) performed prior to EMT revealed extensive CVT. Endovascular mechanical thrombectomy was performed using a large-caliber suction catheter and stent retriever, which is used for arterial thrombectomy in acute large artery occlusion. After EMT, venous outflow of the superior sagittal sinus and both transverse sinuses was completely restored (
Fig. 1E). Although endovascular treatment was successful, the patient became comatose due to extensive brain injury because of severely increased intracranial pressure. On hospital day 8 (day 19 after the vaccination), the result of the PF4 ELISA Ab test was reported as strongly positive (0.72, optical-density units, Lifecodes PF4 IgG assay (Immucor Inc., Norcross, GA, USA) [normal range, < 0.4]). The platelet count did not increase and plasma exchange was initiated; the patient died 20 days after vaccination. He was recognized as the first fatality following and as a result of COVID-19 vaccination in Korea.
 | Fig. 1
Brain CT, MR venogram, and DSA. (A) Axial image of initial non-enhanced brain CT identifies a 5 × 4 × 3-cm3-sized subcortical hematoma in left parietal lobe and an adjacent subarachnoid hemorrhage, which suggests hemorrhagic venous infarction. (B) The lateral view of the MR venogram only demonstrates the left sigmoid sinus and internal jugular vein (arrows), and the remaining dural venous sinus are not visualized. Deep venous drainage systems, such as the internal cerebral vein and great cerebral vein of Galen (arrow heads), can be identified. (C) AP view of DSA performed before mechanical thrombectomy shows multiple filling defects in the right and left transverse sinus (arrows). (D) The lateral view of superior sagittal sinus venography shows a hardly visualized superior sagittal sinus and blockage of the venous outflow to transverse sinus because of extensive thrombosis in the dural venous sinus. Cortical venous reflux due to blockage of venous outflow in superior sagittal sinus was also noted (arrowheads). (E) Lateral view of the superior sagittal sinus venography after endovascular mechanical thrombectomy shows that venous outflow of superior sagittal sinus and transverse sinus was completely restored. (F) A large number of clots which were removed through endovascular mechanical thrombectomy.
CT = computed tomography, MR = magnetic resonance, DSA = digital subtraction angiography, AP = antero-posterior.

|
Table 1
Laboratory results of the patient

|
Laboratory analysis |
Result |
Unit |
Reference value |
|
Hemoglobin |
17.8 |
g/dL |
13.0–18.0 |
|
White blood cell count |
7.40 |
per mm3
|
4.0–10.0 |
|
Platelet count |
14 |
per mm3
|
140–450 |
|
Erythrocyte sedimentation rate |
2 |
mm/h |
0–15 |
|
C-reactive protein |
3.42 |
mg/L |
0–5 |
|
Blood urea nitrogen |
12.5 |
mg/dL |
6.0–20.0 |
|
Creatinine |
0.97 |
mg/dL |
0.70–1.20 |
|
Total protein |
7.3 |
g/dL |
6.6–8.7 |
|
Albumin |
4.3 |
g/dL |
3.4–4.8 |
|
Total bilirubin |
0.91 |
mg/dL |
0.0–1.4 |
|
Aspartate aminotransferase |
14 |
U/L |
0–40 |
|
Alanine aminotransferase |
15 |
U/L |
0–40 |
|
Alkaline phosphatase |
85 |
U/L |
40–129 |
|
Lactate dehydrogenase |
203 |
U/L |
135–225 |
|
Fibrinogen |
77 |
mg/dL |
180–415 |
|
Antithrombin III |
110.7 |
% |
60–120 |
|
Fibrin degradation products |
64.6 |
µg/mL |
< 5.1 |
|
Prothrombin time |
14.0 |
sec |
10.1–14.0 |
|
Activated partial thromboplastin time |
28.0 |
sec |
23.4–30.3 |
|
D-dimer |
> 35.2 |
mg/L |
0–0.55 |
|
Troponin-T |
13.9 |
pg/mL |
0–14 |
|
Creatine kinase-MB |
0.721 |
pg/mL |
0.0–4.87 |







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download