INTRODUCTION
Recent advances in cancer treatment include development of targeted therapies and immune checkpoint inhibitors (ICIs). These chemotherapeutic agents offer clinical benefits such as prolonged survival, less toxicity and improved quality of life (QoL). However, it also entails financial burden for cancer patients and society.
Eight of the top 10 drugs based on total expenditure in 2012 by United State (US) Medicare were anticancer drugs. Approximately, $87.5 billion was spent on cancer treatment in 2012 and the estimated cost for 2020 is $175 billion.
12 Among cancer types, lung cancer has the highest prevalence and the highest cost of treatment at $2.9 billion, which is attributed to the use of expensive drugs such as ICIs in a variety of clinical scenarios.
2
In addition to expanding the market for cancer treatment by adding new indications, ICIs are expected to have a profound effect on expenditure on anticancer drugs by patients as well as the government. Currently, with more than 3,300 new agents in the immuno-oncology pipeline and a wide array of combination regimens under investigation, it can be regarded as an innovation and represents a variety of options from a physician and patient perspective.
3 However, the annual cost of novel anticancer medications routinely exceeds $100,000, which adds to the financial burden at the expense of clinical efficacy.
3
Meanwhile, it has been argued that the value of cancer drugs is not properly measured during the economic evaluation.
45 Since active comparators are frequently lacking in the clinical trials of the cancer drugs, an indirect comparison is unavoidable. The clinical endpoints are often extrapolated based on several statistical techniques, which resulted in substantial uncertainty with the wide range of the Incremental Cost Effectiveness Ratio (ICER). These factors underlying clinical uncertainty and value-based clinical decision making led to the development and adaptation of several value frameworks (VFs), which quantify and evaluate the benefits, harms, and costs. In the past five years, several active discussions revolved around the clinical value of expensive anticancer drugs by diverse organizations and institutions. A few representative value evaluation tools are: European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS),
67 American Society of Clinical Oncology Value Framework (ASCO-VF),
89 National Comprehensive Cancer Network (NCCN) Evidence Block,
10 Drug Abacus Tool by Memorial Sloan Kettering Cancer Center (MSKCC),
11 and the ICER.
12 Incorporating these VFs in chemotherapeutic agent selection by physicians and patients is underway in several discussions of reimbursement issues.
6789101112 These tools make us facilitate the identification of innovative access strategies to specific therapies under clinically diverse scenarios from the perspective of patients, physicians, payers, policymakers, and the pharmaceutical industry. The recent study insists that VFs help to identify therapies providing high clinical benefit and should be made rapidly available across countries.
13
The issues about effective allocation of health resources and implications for value measurement of expensive cancer drugs has emerged from public hearings in Korea, also.
14 Many issues including methodological approaches and the value measurement from each stakeholder’s view make it difficult to reach the consensus. To measure the value of expensive anticancer drugs, a thoughtful analysis by experts is required along with a broad discussion between multiple stakeholders including health insurance, pharmaceutical companies, physicians, and patients.
In this study, we surveyed to explore the perception and attitude for VFs from Korean oncologist’s perspectives, so get insight for the possible implementation of VF for anticancer drugs, simultaneously.
DISCUSSION
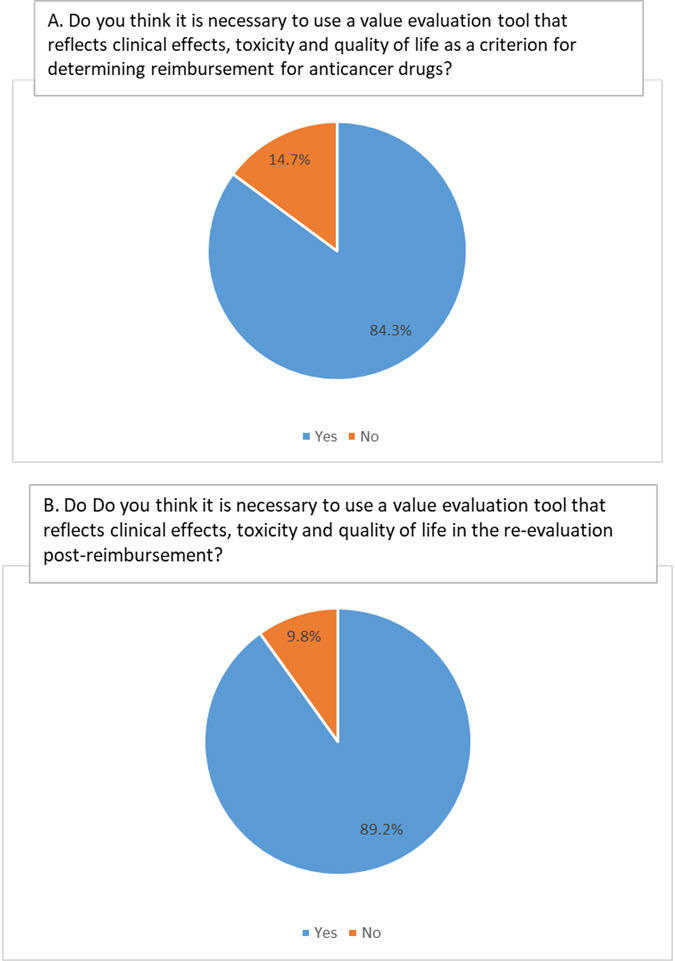
This study showed that the majority of Korean oncologists insisted on the development of VF and its practical application in Korea.
In Korea, total healthcare expense increased between 2015 and 2018, which was mainly attributed to drug acquisition cost.
15 In this proportion, the anticancer drug claim in 2016 was about 1.39 trillion KRW (which equates to US $11.6 billion), accounting for 6.8% of the total drug cost. Additionally, the increase rate was doubled for cancer drugs compared to all drugs and this has been gradually increasing since 2014.
16 The compound annual growth rate (CAGR) of anticancer drug expenditure from 2015 until 2017 was 11.2%, whereas the CAGR for the general pharmaceutical expenditure was 8.2%.
15 These drugs increase the financial burden, which amounted to 233.9 billion KRW (which equates US $194 million) in 2010, increasing annually to 578.5 billion KRW (which equates US $482 million) in 2016.
17
A survey result of 185 cancer patients reported that a total of 28.77 million KRW (which equates US $2.4 thousand) was spent on cancer treatment and anticancer drugs accounted for the highest proportion (60.5%) of the total cost of treatment.
18 When the difficulty of the treatment process is identified, the financial factor was 37.3%, ranking first followed by psychological factor 31.9%, and physical factor 27.6%. The financial burden had worsened while the physical and psychological hardship resolved by passing treatment time.
18
The need for measuring the true value of anticancer drugs is emerging as expensive anticancer drugs, such as ICIs are introduced in clinical field. The Korean scenario is no different than that of the US based on the data of cancer drug expenditure. In fact, our review confirmed the anticancer drugs were the main driver of escalating pharmaceutical expenditure in Korea.
With the introduction of various schemes for enhanced access to expensive anticancer drugs in Korea, such as risk sharing agreement (2014) or waiver of the cost-effectiveness analysis dossiers (2014), it has been reported that the accessibility of the cancer drugs has improved.
19 Nevertheless, the value of expensive anticancer drugs is still uncertain, which underscores the need for VFs in Korea.
ASCO published a VF in 2015 in an effort to incorporate value discussion in the shared decision making between patients and physicians. Briefly, the three variables are clinical benefit, toxicity or side effects of the treatment, and out-of-pocket costs.
67 NCCN evidence blocks were developed by analyzing the scores of NCCN expert panels by plotting onto a 5 × 5 matrix of the panel member's response.
10 As fore mentioned, ICER and MSKCC's Drug Abacus are tools
1112 available for value measurement. These four tools were developed in the US. ESMO-MCBS is unique in that it aims to cover several European nations.
67 Generally, compared with ICER VF tool and Drug Abacus, ASCO-VF and NCCN evidence blocks have been regarded as useful and rated favorably for clinical application by physicians.
2 The key element is that the used or weighted variables differed among these tools. Given the difficulty to estimate the total cost, all the listed VFs do not offer (might not be able to offer) the affordable at the individual patient level. Despite this limitation, VFs provide a comparative assessment of various treatment options available and their relative financial implications for patients.
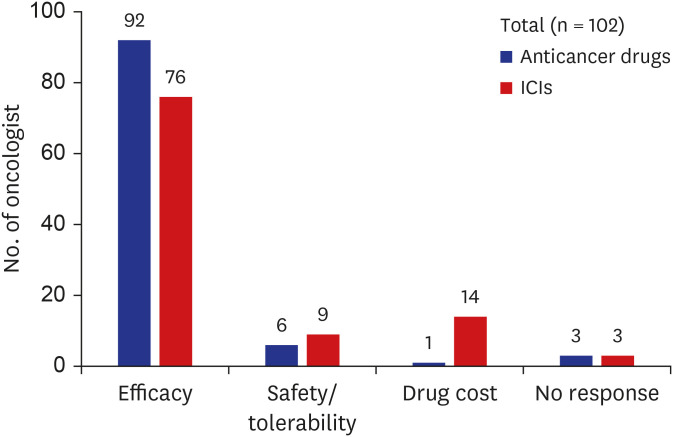
Which parameter does an oncologist usually rank as the highest in priority? More than 90% of respondents gave the answer that improved clinical efficacy such as prolongation of overall survival and/or progression-free survival are the most important factor for determining the drug selection, and only 1% replied that drug cost was the critical factor. Regarding to ICIs, 74.5% replied that clinical efficacy is the top priority and 13.4% responded that drug cost is the key factor. Invariably, improved clinical efficacy was the primary parameter dictating drug selection by medical oncologist and was considered by all VFs as the highest priority despite differences in methodology. Clinical efficacy was also ranked as the highest priority by US physicians, which is consistent with our study.
20 The interviewed US physicians acknowledged that cost/economic factors, system/organization, and social values played a limited role in influencing drug value assessment. However, at the same time, 83% of participants indicated that a patient's affordability was a key determinant of their perception of drug value. We found that our responders heavily considered drug costs associated with ICIs. It appears that Korean oncologists face a conflict between their professional responsibility to provide effective healthcare based on ethical principles of quality and cost-effective medicine.
21 Our findings suggest that the introduction of novel immunoncology drugs with increase of drug price might have influenced the perceived value of the oncology landscape in Korea.
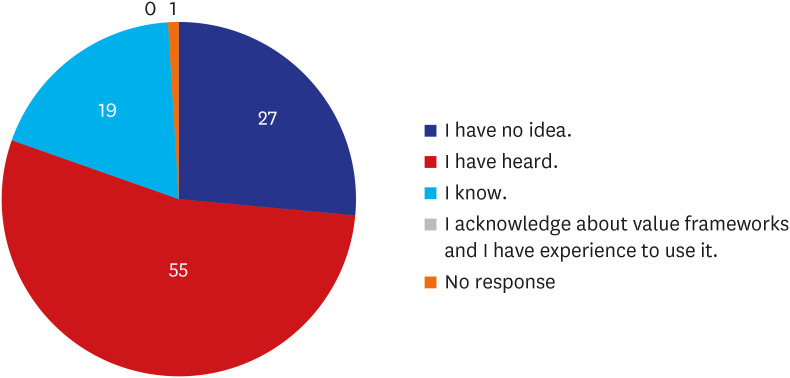
Even though half of the respondents answered that they had heard of VFs previously, none of them experienced clinical use of the VF tools. We compared our findings with two studies conducted with US physicians in 2016 and 2018.
2223 In 2016, 85% of the surveyed respondents (n = 93, medical or hematologic oncologists in community or academic settings) were aware of VFs; however, 46% of survey respondents did not use these tools, which is similar to our finding. A variety of reasons were listed: ‘not mandatory by institution or payer’ (28%), ‘too early to adapt’ (17%), complicated (14%), and ‘don’t worry about cost’ (11%). However, the experience of using VFs increased with 76% (N = 200) of the oncologists in the US using them in 2018
23. The increased usage across two years suggests the need for value measurement and collaboration with key stakeholders in order to adapt VFs to Korean conditions.
The issues implicating the VFs are linked to which VF should be mainly adapted. Even though value-based prescribing is emphasized, physicians responded not to adopt VF until mandatory regulation.
22 Except for clinical decision making between patients and physicians regarding chemotherapeutic agents, the reimbursement list and evaluation of the listing status post-reimbursement are major issues in Korea. In fact, current VFs are increasingly tied to reimbursement strategies, and two VFs, ESMO-MCBS and ICER, are representative because these tools aim for government policy making.
67122324 Generally, the government approves drugs based on clinical trial data, but the setting and design of clinical trials are very different from the real world. We frequently face situations that raise concerns about the clinical efficacy of even high priced drugs. The VFs can be used for reliable analysis of real world data and effective allocation of health resources.
In our analysis, only ASCO-VF and ESMO-MCBS were presented in the survey because the two tools were initiated by global academic societies and are considered clinically representative. According to the survey reported from the ASCO 2016 annual meeting, ASCO-VF are one of the most frequently used VF in the US with 38% response, followed by the NCCN evidence blocks.
22 Because ASCO-VF was intended for use by oncologists as part of the treatment decision-making process, we may consider this tool as the most useful for clinical application. However, the score with meaningful net clinical benefit is confusing since the score is a continuous variable and no clear cut-off information is available. The ESMO-MCBS was acceptable to a multidisciplinary task force from a public health perspective, and its use in reimbursement decisions leads us to benchmark the allocation of health resources in Korea.
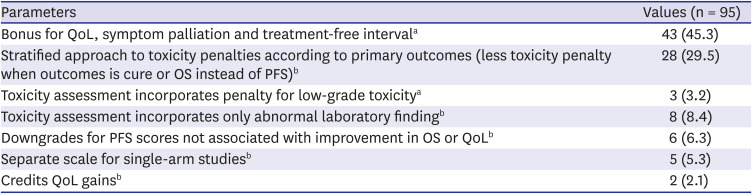
25 Our respondents' answer that both VFs should be reflected for developing Korea VF suggests the consideration for covering the purpose of different uses of the two VFs at the same time. We tried to identify the specific parameters by briefly summarizing the ASCO-VF and ESMO-MCBS for the participants. We focused on the different characteristics of these two VFs because the common parameters are supposed to be included in the Korean context with no doubt. Nearly half of responders selected bonus point as the most important factor when the drug is evaluated based on improved QoL, symptom palliation or treatment-free interval using ASCO-VF. The scoring system of existing VFs is complicated and prevents analysis of our scope using only a one-time survey. Moreover, these tools have their unique shortcomings and methodological limitations of their own.
2526 Despite their intrinsic differences, the two revised versions of VFs, ESMO-MCBS version 1.1 (updated in 2017) and the ASCO-VF version 2.0 (updated in 2016), demonstrated good agreement in scores.
27 Both societies remain committed to refining their framework. The ASCO Value in Cancer Care Task Force composed of physicians sought inputs from an advisory committee that included oncologists, patient advocates, payers and the biopharmaceutical industry, followed by public comments.
28 Suggestions to adjust the inputs or outputs of parameters based on individual patient preferences and consideration for years of life gained or even gain in quality-adjusted life expectancy are being discussed in ASCO.
929 It is unclear how this methodology will ultimately be developed, but we can adapt it by incorporating parameters to conduct further studies to measure the value of cancer treatment reflecting the Korean situation.
This study has some limitations. First, the response was acquired from a proportion of participants in the Best of ASCO and KMMWP study group. Although the opinions of additional oncologists may result in different findings, the response of 100 oncologists was substantial in exploring our study aim. For references, the US studies involved less than 200 oncologists
2223 of approximately 13,000 practicing medical oncologists, with another 500 graduates joining the workforce annually.
30 Second, nearly half of responders never heard of VFs, suggesting that they may not have understood the concept of ASCO-VF and ESMO-MCBS correctly even though we provided a short introduction to the two VFs. Nonetheless, this study is significant in demonstrating the need for value measurement of anticancer drugs from an oncologist perspective with background data of financial burden in Korea for the first time.
In conclusion, we explored the oncologist's perception for possible development and adaptation of VF for anticancer drugs in Korea. In the face of escalating anticancer drug expenditure, VF might represent an appropriate option to relieve financial toxicities occurring from expensive anticancer drugs. Further studies are warranted to get insight into which VF is acceptable or whether an evidence-based VF reflecting the healthcare system can be developed in Korea. Taken together, VF-based strategy is needed to address the needs of multiple stakeholders. We should ensure that cancer patients receive the highest value care within the limited health resources.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download