This article has been
cited by other articles in ScienceCentral.
Abstract
Jaundice is a rare symptom of the paraneoplastic syndrome associated with prostate cancer. We report a case of metastatic prostate cancer that presented as jaundice. There was an absence of biliary obstruction and hepatic metastasis; therefore, the paraneoplastic syndrome was suggested as the etiology of cholestasis. Jaundice improved with the treatment of prostate cancer. In the literature, interleukin-6 has been suggested to be associated with paraneoplastic syndrome.
Go to :

Keywords: Prostatic neoplasms, Cholestasis, Paraneoplastic syndromes, Interleukin-6
INTRODUCTION
An extrahepatic malignant tumor can be associated with cholestasis. When hepatic metastasis, bile duct obstruction, or other causes are ruled out, cholestasis may be attributed to the remote effect of a tumor. This is called paraneoplastic syndrome.
We report a case of a man who presented with paraneo-plastic jaundice as a manifestation of prostate cancer. Cholestasis improved with androgen deprivation therapy.
Go to :

CASE REPORT
An 81-year-old man visited Kangwon National University Hospital with complaints of pruritus and jaundice. He developed pruritus 2 months ago and visited the dermatology department. Subsequently, jaundice was confirmed, and he was referred to department of internal medicine for further evaluation.
He had a weight loss of 9 kg over 2 months and complained of dark urine for the previous 2 weeks. He had no abdominal pain and fever. He had undergone left total hip replacement arthroplasty surgery 9 years ago. There was no other medical history, including hypertension, diabetes, and chronic liver disease.
He was a current smoker (40 pack-years) and had stopped drinking alcohol 1 year ago. He did not take any toxic sub-stances or herbal medication.
Physical examination revealed icteric sclera, and there was no mass or tenderness observed on abdominal examination. The results of laboratory tests were as follows: white blood cell count 6,800/μL (reference range 3.8-10.0×103/μL), hemoglobin 12.1 g/dL (13.3-16.5 g/dL), platelet count 529,000/μL (140-400×103/μL), total bilirubin 23.0 mg/dL (0.3-1.2 mg/dL), direct bilirubin 16.84 mg/dL (0-0.40 mg/dL), GGT 81 U/L (0-73 U/L), AST 50 U/L (0-34 U/L), ALT 32 U/L (10-49 U/L), ALP 678 U/L (45-129 U/L), and CRP 0.524 mg/dL (0-0.500 mg/dL). BUN, creatinine, sodium, potassium, chloride, glucose level, and prothrombin time were within their normal limits. The serologic tests for viral hepatitis were negative. Anti-nuclear and mitochondrial antibodies were negative. The ceruloplasmin level was 42.0 mg/dL (15.0-30.0 mg/dL) and the IgG level was 783 mg/dL (700-1,600 mg/dL).
CT did not reveal a hepatic mass, bile duct dilatation, or obstruction (
Fig. 1A). It showed a heterogeneous enhancement of the prostate, lymph node enlargement in bilateral iliac chains, and diffuse presence of osteoblastic lesions in the axial skeleton, which were suggestive of prostate cancer with metastasis (
Fig. 1B). The whole body bone scan with technetium-99m was suggestive of multiple bone metastases (
Fig. 1C).
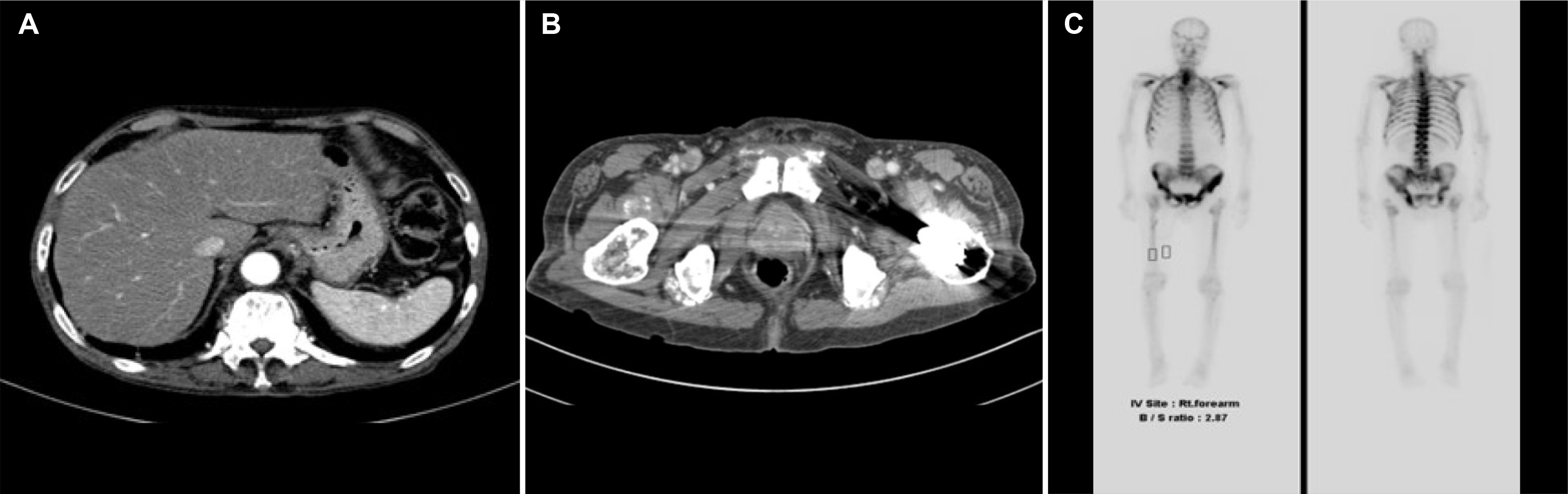 | Fig. 1Computed tomography showed (A) no findings of metastasis, hepatic infiltration, and no bile duct dilation or obstruction (B) heterogeneous enhancement of the prostate. (C) The whole body bone scan was suggestive of multiple bone metastases. 
|
The serum prostate-specific antigen (PSA) level was elevated (59.68 ng/mL, reference range 0-4 ng/mL), and the carbohydrate antigen 19-9 level was within the reference range. Ultrasonography-guided prostate biopsy revealed adenocarcinoma. The Gleason score was 7 (4+3) (
Fig. 2A). A liver biopsy was also performed, and it showed marked cholestasis and nodular inflammation. However, fatty change, interface hepatitis, bile duct damage, granuloma, and malignant cell infiltration were not present (
Fig. 2B).
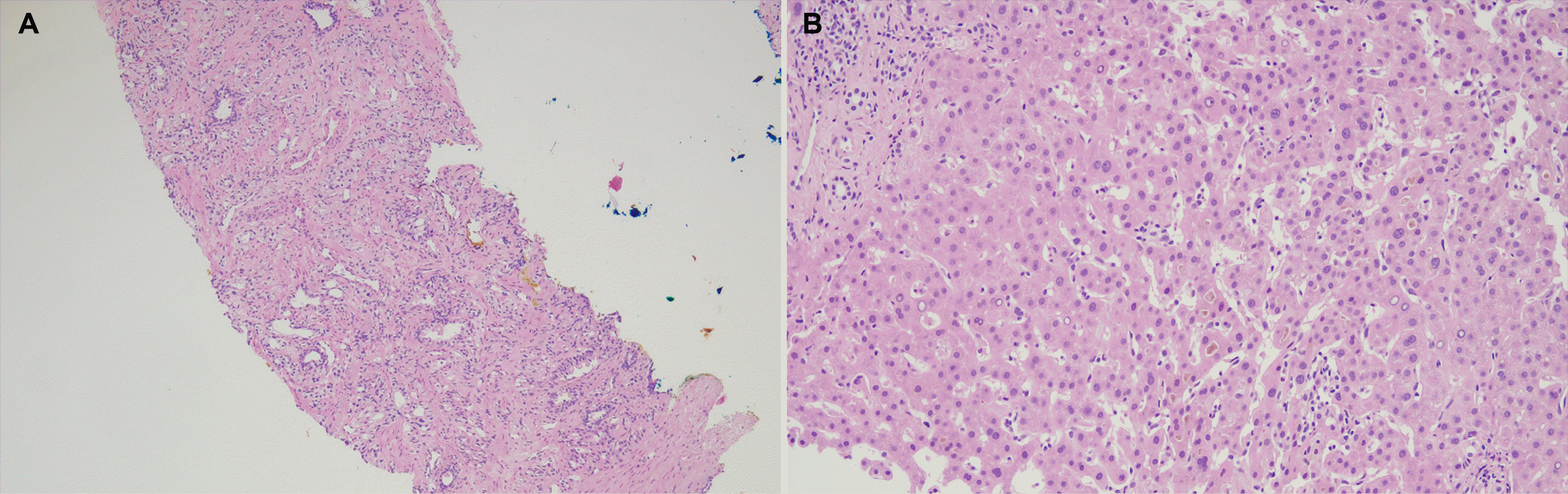 | Fig. 2(A) Prostate biopsy revealed adenocarcinoma (hematoxylin and eosin stain, ×100). (B) Liver biopsy showed cholestasis and nodular inflammation (hematoxylin and eosin stain, ×200). 
|
PET/CT revealed diffuse osteosclerotic change with hyper-metabolism along the axial and appendicular skeletons, which was suggestive of metastasis. However, no abnormal hyper-metabolic lesion was found in the liver and biliary tree.
We made the diagnosis of paraneoplastic jaundice with prostate cancer. From the 14th day of hospitalization, the patient was started on leuprorelin 22.5 mg subcutaneous injections, (to be adimistered every 3 months) and oral bicalutamide 50 mg daily. However, hepatotonics were not prescribed. Total bilirubin and ALT levels gradually decreased. The ALP level decreased initially, but it increased again. After the second leuprorelin injection, the ALP level finally decreased. The PSA level rose to 3,319.91 ng/mL after 2 weeks, and then it decreased to 2,260 ng/mL at the time of the second leuprorelin injection (
Table 1,
Fig. 3). Contrast-enhanced MRI of the prostate was performed three weeks after the first leuprorelin injection. It revealed diffuse involvement of prostate cancer in both lobes of the prostate gland. Other follow-up imaging tests were not performed.
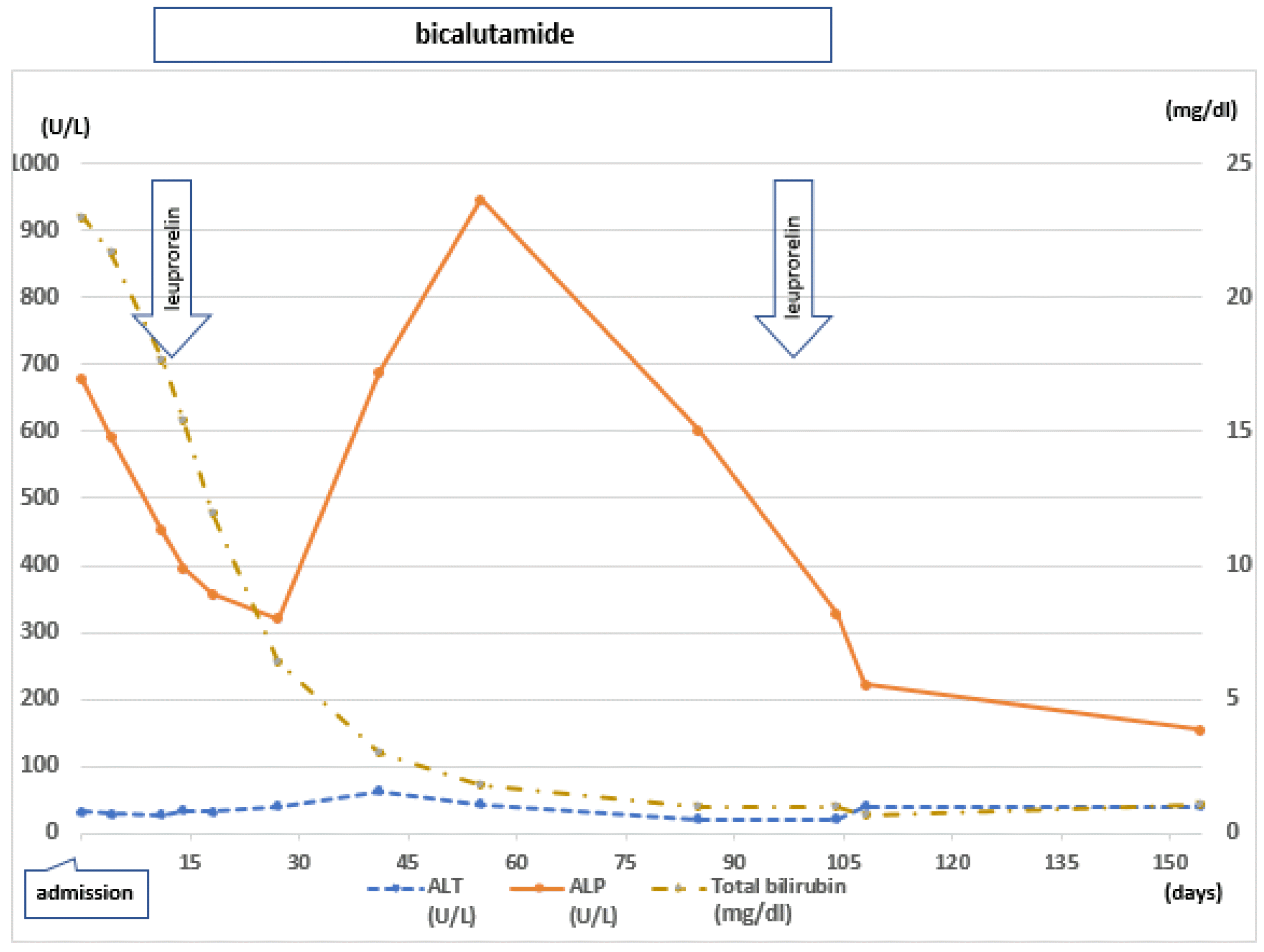 | Fig. 3Clinical course of the patient. From the 14th day of hospitalization, leuprorelin was injected every 3 months, and bicalutamide was administered daily. Total bilirubin level gradually decreased. 
|
Table 1
Interval Change of the Laboratory Findings
|
On admission |
4th day |
11th day |
14th day |
18th day (discharge) |
27th day |
41th day |
55th day |
85th day |
104th day |
108th day |
|
|
1st leuprorelin SC |
|
|
|
|
|
2nd leuprorelin SC |
|
|
|
Oral bicalutamide administered daily |
|
Total bilirubin (mg/dL) |
23 |
21.7 |
17.7 |
15.4 |
12 |
6.4 |
3 |
1.8 |
1 |
1 |
0.7 |
|
Direct bilirubin (mg/dL) |
16.84 |
16.76 |
14.32 |
12.53 |
9.82 |
6.17 |
2.39 |
1.31 |
0.39 |
0.39 |
|
|
AST (U/L) |
50 |
44 |
50 |
57 |
54 |
64 |
83 |
49 |
37 |
31 |
68 |
|
ALT (U/L) |
32 |
29 |
28 |
33 |
32 |
41 |
63 |
44 |
21 |
21 |
40 |
|
ALP (U/L) |
678 |
593 |
453 |
396 |
357 |
320 |
689 |
946 |
602 |
328 |
222 |
|
GGT (U/L) |
81 |
78 |
63 |
64 |
77 |
146 |
350 |
374 |
200 |
134 |
|
|
PSA (ng/dL) |
60 |
|
|
|
|
3,320 |
|
|
|
2,260 |
|

Four days after the second leuprorelin injection, the patient was readmitted due to myocardial infarction and was managed with percutaneous coronary intervention. He did not have any hepatobiliary problem during hospitalization and was discharged 12 days later. After 2 months, he suffered a cardiac arrest while working in the field and expired.
Go to :

DISCUSSION
This is a case of a metastatic prostate cancer patient presenting with jaundice and pruritus. Jaundice showed a choles-tatic pattern; however, the etiology was unclear. There was an absence of extrahepatic biliary obstruction. Viral, toxic hepatitis, and other rare causes were also excluded. Based on the imaging based diagnostic tests, we concluded that cholestasis was paraneoplastic jaundice associated with metastatic prostate cancer.
Stauffer’s syndrome is a rare paraneoplastic syndrome, which is characterized by cholestasis, in which the patient may present with pruritus, jaundice, elevated liver enzymes, hepatosplenomegaly without liver metastases, bile duct obstruction, and other hepatocellular disorders. It disappears when the tumor is controlled.
1 This disease entity was first reported in renal cell carcinoma (RCC) by an American gastroenterologist Maurice H. Stauffer. Prostate cancer, soft tissue sarcoma, pancreatic cancer, bladder cancer, malignant lymphoproliferative diseases, bronchogenic carcinoma, and gastrointestinal neuroendocrine tumor have also been reported to be associated with this syndrome. Prostate cancer is the second most common malignancy associated with paraneoplastic jaundice.
2
However, Stauffer’s syndrome has been rarely reported in prostate cancer. A thorough search of medical literature revealed only 17 case reports published in the english language (
Table 2).
1,3-18 Of these, 13 cases were of newly diagnosed prostate cancer, and cholestasis which improved after treatment, and five cases where improvement was not seen. There was accompanying distant metastasis in all cases. To the best of our knowledge, this case is the second reported case of prostate cancer with Stauffer’s syndrome in South Korea.
Table 2
Summary of Cases of Paraneoplastic Cholestasis in Prostate Cancer
|
Study |
Age (years) |
Total bilirubin (mg/dL) |
ALP (U/L) |
PSA (ng/dL) |
Liver biopsy |
Prostate cancer |
Treatment |
Outcome (cholestasis) |
|
Reddy et al. (1977)3
|
57 |
6.2 |
1,835 |
Unknown |
Fibrosis, inflammatory infiltration, cholestasis |
Known |
Radiation, diethylstilbestrol |
Improved |
|
Karakolios et al. (2003)4
|
72 |
18.1 |
4,679 |
150 |
No |
ND |
Flutamide, leurpolide |
Improved |
|
Koruk et al. (2004)5
|
77 |
10 |
5,631 |
100 |
Normal |
ND |
Goserelin, bicalutamide |
Improved |
|
Shah (2006)6
|
64 |
9 |
86 |
643.8 |
No |
ND |
Cypoterone |
Not Improved |
|
Nguyen et al. (2011)7
|
51 |
16 |
579 |
556 |
Normal |
ND |
Goserelin, bicalutamide |
Improved |
|
Hinostroza-Yanahu aya et al. (2013)1
|
70 |
29 |
1,713 |
1,548 |
No |
Known |
Goserelin, estramustine |
Not improved |
|
84 |
3.96 |
581 |
WNL |
No |
ND |
Leuprolide |
Not improved |
|
Kuramoto et al. (2013)8
|
75 |
17 |
44 |
9,862 |
No |
ND |
Leuprolide, bicalutamide |
Improved |
|
Okano et al. (2014)9
|
68 |
23.4 |
2,182 |
15,018 |
Inflammation, cholestasis |
ND |
Bicalutamide |
Improved |
|
Kato et al. (2014)10
|
60 |
9.3 |
1,803 |
41.4 |
No |
ND |
Bicalutamide, leuprolide |
Improved |
|
Walter et al. (2015)11
|
72 |
8.2 |
4,364 |
1,061 |
Cholestasis, hematopoiesis |
Known |
Leuprolide |
Improved |
|
Vieira et al. (2017)12
|
78 |
12.3 |
950 |
>1,000 |
No |
ND |
Bicalutamide, goserelin |
Improved |
|
Bhangoo et al. (2018)13
|
67 |
26.3 |
988 |
4,130 |
No |
ND |
Leuprolide, bicalutamide |
Improved |
|
Ravindranathan et al. (2018)14
|
47 |
15.3 |
524 |
>1,300 |
Inflammation, cholestasis |
ND |
Degarelix, leuprolide, doxetalel |
Improved |
|
Kang et al. (2018)15
|
72 |
13.1 |
Unknown |
7,895 |
Cholestasis |
ND |
prednisone, Bicalutamide, goserelin |
Improved |
|
Romašovs et al. (2019)16
|
80 |
165 |
2,513 |
66.01 |
No |
Known |
ADT |
Not Improved |
|
Liu et al. (2019)17
|
88 |
3.8 |
654 |
3,030 |
No |
Known |
Enzalutamide |
Not Improved |
|
Gökçen et al. (2018)18
|
65 |
14.14 |
600 |
>150 |
No |
ND |
Bicalutamide, goserelin |
Improved |
|
Present case |
81 |
23 |
678 |
59.68 |
Inflammation, cholestasis |
ND |
Leurprorelin, bicalutamide |
Improved |

The pathophysiology of Stauffer’s syndrome is unknown. An association between IL-6 and paraneoplastic manifestations has been observed in RCC, including cholestasis, although the causality is less well established.
2 The association between IL-6 and cholestasis may be mediated by systemic inflammation. Pro-inflammatory cytokines, including IL-6, are involved in the up-regulation of inflammation and inhibition of the expression of the hepatobiliary transporter gene possibly accounting for biliary outflow.
13
In a study, the anti-IL-6 monoclonal antibody was found to reverse most of the biochemical abnormalities in patients with Stauffer’s syndrome with RCC.
19 Kuramoto et al.
8 reported that the serum IL-6 levels decreased after anti-androgen therapy. Unfortunately, IL-6 was not measured in this case. The correlation between the IL-6 level and cholestasis needs further investigation.
Stauffer’s syndrome is characterized by paraneoplastic cholestasis, which is rarely seen in prostate cancer. It is necessary to consider the possibility of paraneoplastic cholestasis in cases of prostate cancer with cholestasis of unknown etiology.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download