INTRODUCTION
Liver transplantation (LT) is a life-saving procedure for patients with end-stage liver diseases. LT is limited in many countries due to the shortage of organ donors, high medical cost, and long waiting lists. Patients in countries with a low incidence of deceased donors and in which living donor liver transplantation (LDLT) has not been developed have sought alternative methods to undergo LT [
1]. Many patients in these countries have undergone LT in other countries. The Korean Network for Organ Sharing (KONOS) has reported that 162 overseas non-Korean patients underwent LT in Korea between 2006 and 2016; of these, 39% were from Mongolia, 23% from the United Arab Emirates, 13% from China, and 6% from the United States [
2].
Overseas travel for organ transplantation is different from transplant tourism because the former consists of visiting highly suitable institutions in other countries, usually highly developed LDLT centers, for living donor organ transplantation. LDLT for foreign patients is associated with several highly sensitive issues, including the possibility of organ trafficking and transplant tourism, the perioperative safety of recipients and donors, the management of LT-associated complications, and the posttransplant follow-up [
1]. To overcome these issues, it is necessary to develop a standard framework for overseas LDLT, as suggested by the Declaration of Istanbul [
3,
4]. The present study describes the institutional standard framework and experience of LDLT for overseas non-Korean patients in a Korean high-volume LDLT center.
Go to :

METHODS
The study protocol was approved by the Institutional Review Board at Asan Medical Center (IRB No. 2020-0836), which waived the requirement for informed consent due to the retrospective nature of this study. This study was performed in accordance with the ethical guidelines of the World Medical Association Declaration of Helsinki 2013.
Study Design and Patient Selection
This was a retrospective observational study. The LT database at our institution was searched to identify patients of non-Korean nationality who underwent LDLT over a 10-year period from January 2010 to December 2019. During this study period, 3,401 patients underwent LDLT at our institution; of these, 105 (3.1%) were overseas non-Korean patients who had traveled for LDLT. The medical records of these patients were retrospectively reviewed, with patient follow-up until August 2020.
Statistical Analysis
Numerical data are presented as means with standard deviation. Continuous variables were compared using the Student t-test or Mann-Whitney U-test, and categorical variables were compared using the chi-square test. Survival rates were estimated using the Kaplan-Meier method and compared with the log-rank test. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS ver. 22 (IBM Corp., Armonk, NY, USA).
Go to :

RESULTS
Institutional Standard Framework for LDLT in Overseas Non-Korean Patients
The institutional standard framework for LDLT was the same in foreign patients and Korean citizens [
5-
9]. All recipient and donor candidates underwent routine pretransplant evaluation (
Tables 1 and
2). All processes for diagnosis and treatment of patients and donors were performed with the assistance of institutional coordinators who spoke their languages. Our international healthcare center has in-house native-speaking coordinators for English, Japanese, Russian, Arabic, Vietnamese and Mongolian languages. Organ donations were formally approved according to KONOS regulations and the Organ Transplant Act in Korea. The documents, translated into Korean, were submitted to KONOS for approval of LDLT. Posttransplant patient care included communications with the medical professionals in the patient’s home country.
Table 1
Pretransplant work-up for recipient candidates
|
Group |
Item |
|
General blood test |
ABO-Rh typing, complete blood count, coagulation battery, electrolyte battery, blood urea nitrogen, creatinine, AST, ALT, total bilirubin, calcium, phosphorus, albumin, amylase, lipase, ammonia, lactic acid, HbA1c
Lymphocyte subset, isoagglutinin titer (for ABO-incompatible transplantation) |
|
Serologic test |
Hepatitis B virus, hepatitis C virus, hepatitis A virus, human immunodeficiency virus, syphilis, cytomegalovirus, Epstein-Barr virus |
|
Tumor marker test |
AFP, PIVKA-II, CEA, CA19-9 |
|
Routine imaging study |
Dynamic abdomen-pelvis CT, chest CT, contrast liver MR, hepatic mesenteric Doppler, bone scan, positron emission tomography, brain MR |
|
Endoscopic examination |
Esophagogastroduodenoscopy, sigmoidoscopy (colonoscopy) |
|
Heart and lung work-up |
Electrocardiography, echocardiography, thallium SPECT, pulmonary function test, chest X-ray, coronary artery CT |
|
Urine test |
Urinalysis |
|
Stool test |
Parasite |
|
Culture |
Blood, sputum, urine |
|
Multidisciplinary consultation |
Dentistry, otolaryngology, ophthalmology, psychiatry, social welfare |

Table 2
Pretransplant workup for living donors
|
Group |
Item |
|
General blood test |
ABO-Rh typing, complete blood count, coagulation battery, electrolyte battery, blood urea nitrogen, creatinine, AST, ALT, total bilirubin, calcium, phosphorus, albumin, amylase, lipase, ICG-R15 |
|
Serologic test |
Hepatitis B virus, hepatitis C virus, hepatitis A virus, human immunodeficiency virus, syphilis, cytomegalovirus, Epstein-Barr virus |
|
Routine imaging study |
Dynamic liver CT with volumetry, liver MR with cholangiography, hepatic mesenteric Doppler |
|
Fatty liver evaluation |
Ultrasonography-guided needle liver biopsy |
|
Heart and lung work-up |
Electrocardiography, echocardiography, thallium SPECT, pulmonary function test, chest X-ray, coronary artery CT |
|
Urine test |
Urinalysis, human chorionic gonadotropin |
|
Stool test |
Parasite |
|
Bacteria culture |
Blood, sputum, urine |
|
Multidisciplinary consultation |
Psychiatry, social welfare |

Profiles of Overseas Non-Korean Patient LDLT
During the 10-year period from 2010 to 2019, 105 overseas non-Korean patients underwent LDLT at our institution; their nationalities are summarized in
Table 3. Of these 105 patients, 88 (83.8%) were from the United Arab Emirates and Mongolia. Profiles of the recipients and donors from overseas countries are summarized in
Table 4. Of these 105 recipients, 95 (90.5%) were adults; their most common primary diseases were hepatitis B and C virus-associated liver cirrhosis. Their mean Model for End-Stage Liver Disease (MELD) and Child-Turcotte-Pugh (CTP) scores were 16.9±8.2 and 8.4±1.9, respectively. Seven patients were ABO-incompatible with their donors, thus requiring pretreatment with rituximab and exchange plasmapheresis. Four patients had previously undergone LT in other countries; thus, these patients were undergoing their second LT operation in our institution. Right liver grafts were implanted into 78 patients. The mean hospital stays before and after LDLT were 8.3±7.4 days and 39.8±32.1 days, respectively. Sixteen patients underwent dual-graft LDLT, resulting in a total of 111 living donors for these 95 adult recipients. Of these donors, 49.6% were the sons and daughters of recipients. Postoperative hospital stay of donors was 12.1±4.4 days.
Table 3
Nationalities of patients who underwent living donor liver transplantation at Asan Medical Center by year of transplantation
|
Country of nationality |
2010 |
2012 |
2013 |
2014 |
2015 |
2016 |
2017 |
2018 |
2019 |
Subtotal |
|
United Arab Emirates |
0 |
2 |
6 |
7 |
12 |
6 |
5 |
8 |
12 |
58 |
|
Mongolia |
1 |
6 |
3 |
4 |
2 |
2 |
5 |
7 |
0 |
30 |
|
China |
0 |
0 |
0 |
1 |
0 |
0 |
2 |
0 |
2 |
5 |
|
Vietnam |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
1 |
2 |
4 |
|
Israel |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
2 |
|
United States |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
|
Singapore |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
|
Russia |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
|
Kazakhstan |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
1 |
|
Chile |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
|
Kuwait |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
|
Subtotal |
1 |
9 |
10 |
12 |
15 |
11 |
12 |
17 |
18 |
105 |

Table 4
Profiles of the recipients and donors from overseas countries
|
Variable |
Adult LDLT (n=95) |
Pediatric LDLT (n=10) |
P-value |
|
Recipient |
|
|
|
|
Age (yr) |
52.5±12.1 |
5.6±4.4 |
<0.001 |
|
Male:female |
58:37 |
5:5 |
0.50 |
|
MELD/PELD score |
16.9±8.2 |
13.5±7.6 |
0.25 |
|
CTP score |
8.4±1.9 |
8.1±1.9 |
0.81 |
|
Country of nationality |
|
|
NA |
|
United Arab Emirates |
50 |
8 |
|
|
Mongolia |
29 |
1 |
|
|
China |
5 |
|
|
|
Vietnam |
4 |
|
|
|
Others |
7 |
1 |
|
|
Diagnosis |
|
|
NA |
|
HBV-LC |
38 |
|
|
|
HCV-LC |
26 |
|
|
|
NBNC-LC |
19 |
|
|
|
ALD-LC |
5 |
|
|
|
AIH-LC |
2 |
|
|
|
PSC |
3 |
1 |
|
|
NASH |
2 |
|
|
|
Biliary atresia |
|
3 |
|
|
Acute liver failure |
|
2 |
|
|
Liver malignancy |
|
2 |
|
|
Genetic metabolic disease |
|
2 |
|
|
Type of graft |
|
|
NA |
|
Right liver graft |
78 |
|
|
|
Dual grafts |
16 |
|
|
|
Left liver graft |
1 |
7 |
|
|
Left lateral section graft |
|
3 |
|
|
ABO-incompatibility |
7 |
0 |
|
|
Hospital stay (day) |
|
|
0.49 |
|
Pretransplant |
8.3±7.4 |
4.1±2.2 |
0.13 |
|
Posttransplant |
39.8±32.1 |
28.8±15.0 |
0.31 |
|
Recipient-donor relationship |
|
|
NA |
|
Parent |
|
8 |
|
|
Son/daughter |
55 |
|
|
|
Sibling |
12 |
1 |
|
|
Uncle/aunt |
6 |
1 |
|
|
Nephew/niece |
18 |
|
|
|
Cousin |
4 |
|
|
|
Other relative |
8 |
|
|
|
Spouse |
8 |
|
|
|
Donor |
|
|
|
|
Age (yr) |
29.1±7.9 |
33.2±9.2 |
0.13 |
|
Male:female |
76:35 |
6:4 |
0.41 |
|
Postoperative hospital stay (day) |
12.1±4.4 |
11.0±1.7 |
0.50 |

Of the 10 pediatric recipients, eight were from the United Arab Emirates; their common primary diseases were biliary atresia, acute liver failure, hepatoblastoma, and genetic metabolic diseases. Their Pediatric End-Stage Liver Disease (PELD) and CTP scores were 13.5±7.6 and 8.1±1.9, respectively. Left liver grafts were implanted into seven patients. Their mean hospital stays before and after LDLT were 4.1±2.2 days and 28.8±15.0 days, respectively. Of the 10 recipients, eight received liver grafts from their parents. Postoperative hospital stay of donors was 11.0±1.7 days.
Posttransplant Outcomes
None of the recipients died in hospital within 3 months. Three of the adult recipients, however, died in hospital at 137, 197, and 220 days, respectively, after LDLT owing to sepsis. The remaining 92 adult patients were discharged from our institution. During posttransplant follow-up, one patient was readmitted to our institution due to pneumonia, but she died of uncontrolled pneumonia. None of the pediatric recipients died in hospital, and all returned to their native countries.
The 1-, 3-, and 5-year overall patient survival rates were 96.2%, 92.4%, and 92.4%, respectively, in all 105 patients; 95.8%, 91.3%, and 91.8%, respectively, in the 95 adult recipients; and 100%, 100%, and 100%, respectively, in the 10 pediatric recipients (P=0.47) (
Fig. 1). All 121 living donors recovered and were discharged without complications or sequelae. Clavien-Dindo grade II surgical complications occurred in eight donors (6.6%). In addition, one donor underwent reoperation due to severe stenosis of the hepatic vein resection site (Clavien-Dindo grade IIIb). The underlying cause of stenosis at the donor inferior vena cava was presumed to be deep side clamping of the right hepatic vein in a donor with the relatively small-sized inferior vena cava. Thus, the incidence of major donor complications was 0.8% (1/121).
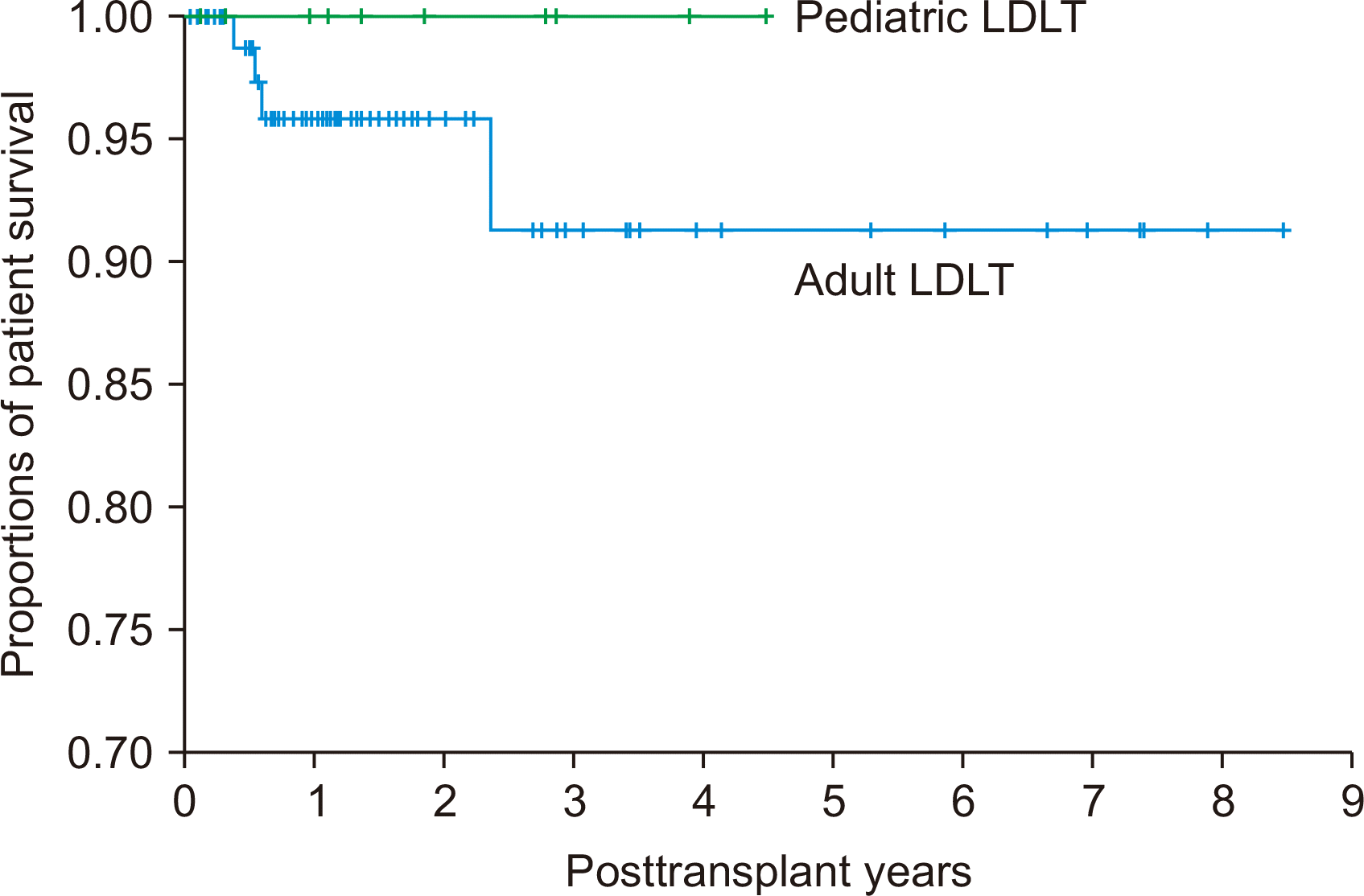 | Fig. 1Kaplan-Meier analysis of the posttransplant patient survival following living donor liver transplantation (LDLT) in adults and children. 
|
Go to :

DISCUSSION
Traveling abroad for cost-effective or high-quality medical care is not uncommon in many countries. Cosmetic plastic surgery is one of the most common reasons for medical tourism. Unlike such cosmetic surgery, solid organ transplantation has many serious aspects, including the socio-ethical issues regarding organ trafficking and transplant tourism and the medical issues regarding patient safety.
The United Network for Organ Sharing (UNOS) has defined transplant tourism as the purchase of transplant organs abroad, including access to organs while bypassing the laws, rules, or process of any or all countries involved [
10]. The Istanbul Declaration [
3,
4] differentiated transplant tourism from travel for transplantation, which has been defined as the movement of organs, donors, recipients, or transplant professionals across jurisdictional borders for transplantation purposes. Transplant tourism has many negative connotations. The Korean Society for Transplantation surveyed all Korean citizens who traveled overseas for organ transplantation, finding that these recipients received suboptimal quality of care [
11]. Between 2000 and 2016, 1,205 Koreans underwent LT in China, 22 in the United States, and one each in Singapore and India [
3]. Koreans who traveled to other countries had lower success rates and were at a higher risk for transmission of infectious diseases than those who underwent transplant in Korea [
11-
14]. Traveling overseas can also result in poor communications between doctors and patients because of the language barrier; moreover, documents may not be properly endorsed after the procedure [
11,
14]. Despite these issues, the medical community has a moral obligation to provide standard medical care to all patients based on the principles of nonjudgmental regard, beneficence, and fiduciary responsibility [
14].
LDLT performed on foreign patients in Korean transplant centers differs from transplant tourism. First, Korean legislation for human organ transplantation is very strict, which effectively prevents organ trafficking. In Korea, KONOS regulations and the Organ Transplant Act permit living donor organ donation only between close relatives by blood or marriage, rules that apply equally to Korean citizens and foreigners. Consent of the biologic parents of the living donor was essential to approve live organ donation in Korea regardless of nationality. All documents translated in Korean are submitted to KONOS for formal approval of foreigner LDLT. The approval process by KONOS is same for both Korean citizens and foreigners. Second, foreign patients and their families are in constant communication with institutional coordinators, who can speak the patient’s language, regarding the patient’s condition and progress. These coordinators also provide the medical abstracts of physicians when the patient returns home. Contact with the medical staff in the patient’s home country is essential to bridge the communication gap. These standard frameworks for foreign patient LDLT are similar in Korea and Taiwan [
15].
Patients around the world are encouraged to travel for LDLT to Asian countries with experience in these operations probably because of high-quality outcomes and medical cost-effectiveness. Because the LT program at our center has performed more LDLTs than any other institution in the world, a considerable number of overseas non-Korean patients, who would have difficulties undergoing usual LDLT, have visited our institution. Usual LDLT might have been precluded in these patients by complicated recipient conditions or donor problems requiring dual-graft LDLT. The most common reason for patients to visit our institution for LDLT was that this operation was not feasible in their home countries. These patients therefore sought high-quality LDLT rather than cost-effective treatment. None of the recipients in this study died in hospital within 3 months. Of the 95 adult recipients, 16 (16.8%) received dual-graft LDLT, suggesting that these patients lacked a suitable single living donor and had visited our institution specifically to undergo dual-graft LDLT. We found that only three of 10 pediatric patients underwent LDLT for biliary atresia. By contrast, of 43 foreign pediatric patients who underwent LDLT at Kaohsiung Chang Gung Memorial Hospital, 39 had biliary atresia [
1]. This finding indicates that pediatric patients requiring usual LDLT did not preferentially visit our institution. Several high-volume centers in Taiwan, Singapore, and India provide lower-cost LDLT as part of their medical brokerage business [
16]. Nevertheless, medical cost-effectiveness is one of the important factors to choose the LDLT center for foreign patients.
The majority of our non-Korean patients were from Mongolia and the United Arab Emirates. Because hepatitis C virus infection is prevalent in Mongolia, many patients have liver cirrhosis. However, LT has not been developed in Mongolia, resulting in a considerable number of patients who have visited Korea for LDLT. The United Arab Emirates has provided financial support to patients and their family members to travel overseas for life-saving medical treatments, including LT, when specific treatments are not available in their home country.
Establishment of a peri-transplant care system in the patients’ countries is essential to permit overseas organ transplantation. Tens of transplant surgeons and physicians from Mongolia, the Middle East, and South Asia have trained in our institution as clinical fellows through an international assistance program at our institution, called “Asan in Asia”, and then returned to their home countries [
17]. These professionals have greatly contributed to caring for patients from their home countries who have undergone overseas LT.
This study has a limitation of note. From the standpoint point of domestic medical service provider, posttransplant follow-up of these non-Korean LT recipients was limited because they returned to their home countries soon after discharge from hospital, and only a small percentage has regularly visited our institution for long-term follow-up.
In conclusion, LDLT at Korean high-volume LT centers, including our institution, is safe and effective for overseas non-Korean patients with end-stage liver disease seeking alternatives not available in their home countries. Our institutional framework for LDLT in foreign patients is effective in preventing organ trafficking and transplant tourism, as well as in providing high-quality medical care. These findings suggest the need to develop international guidelines for overseas LDLT.
Go to :





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download