1. Mangus RS, Fridell JA, Vianna RM, Cooper AB, Jones DT, Tector AJ. 2008; Use of the piggyback hepatectomy technique in liver transplant recipients with hepatocellular carcinoma. Transplantation. 85:1496–9. DOI:
10.1097/TP.0b013e31816feec0. PMID:
18497692.

2. Moon DB, Lee SG, Hwang S, Kim KH, Ahn CS, Ha TY, et al. 2013; No-touch en bloc right lobe living-donor liver transplantation with inferior vena cava replacement for hepatocellular carcinoma close to retrohepatic inferior vena cava: case report. Transplant Proc. 45:3135–9. DOI:
10.1016/j.transproceed.2013.08.052. PMID:
24157050.

3. Matsuda H, Sadamori H, Shinoura S, Umeda Y, Yoshida R, Satoh D, et al. 2010; Aggressive combined resection of hepatic inferior vena cava, with replacement by a ringed expanded polytetrafluoroethylene graft, in living-donor liver transplantation for hepatocellular carcinoma beyond the Milan criteria. J Hepatobiliary Pancreat Sci. 17:719–24. DOI:
10.1007/s00534-010-0287-z. PMID:
20425126.

4. Smyrniotis VE, Kostopanagiotou GG, Gamaletsos EL, Vassiliou JG, Voros DC, Fotopoulos AC, et al. 2002; Total versus selective hepatic vascular exclusion in major liver resections. Am J Surg. 183:173–8. DOI:
10.1016/S0002-9610(01)00864-9.

5. Azoulay D, Lim C, Salloum C, Andreani P, Maggi U, Bartelmaos T, et al. 2015; Complex liver resection using standard total vascular exclusion, venovenous bypass, and in situ hypothermic portal perfusion: an audit of 77 consecutive cases. Ann Surg. 262:93–104. DOI:
10.1097/SLA.0000000000000787. PMID:
24950284.
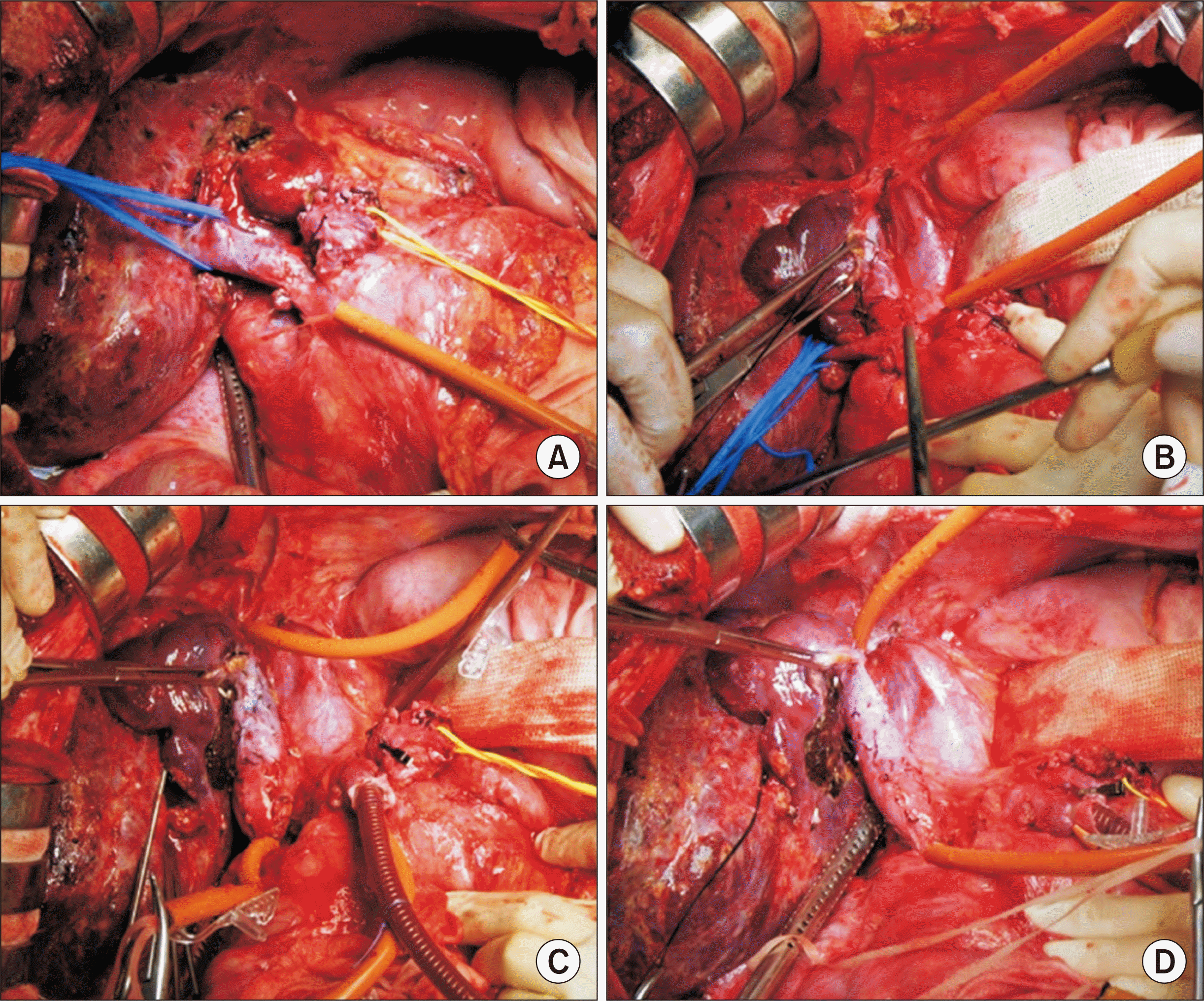
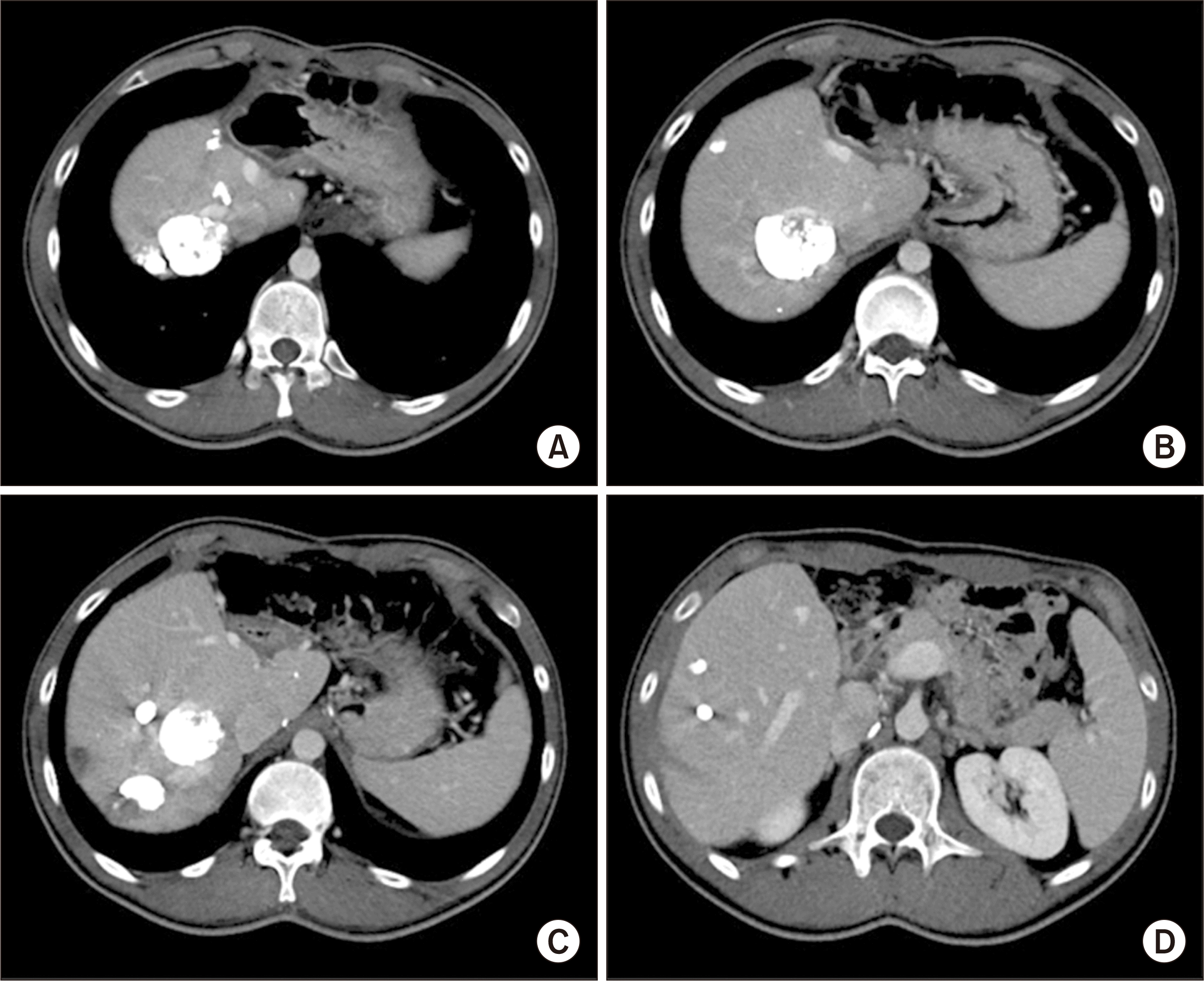
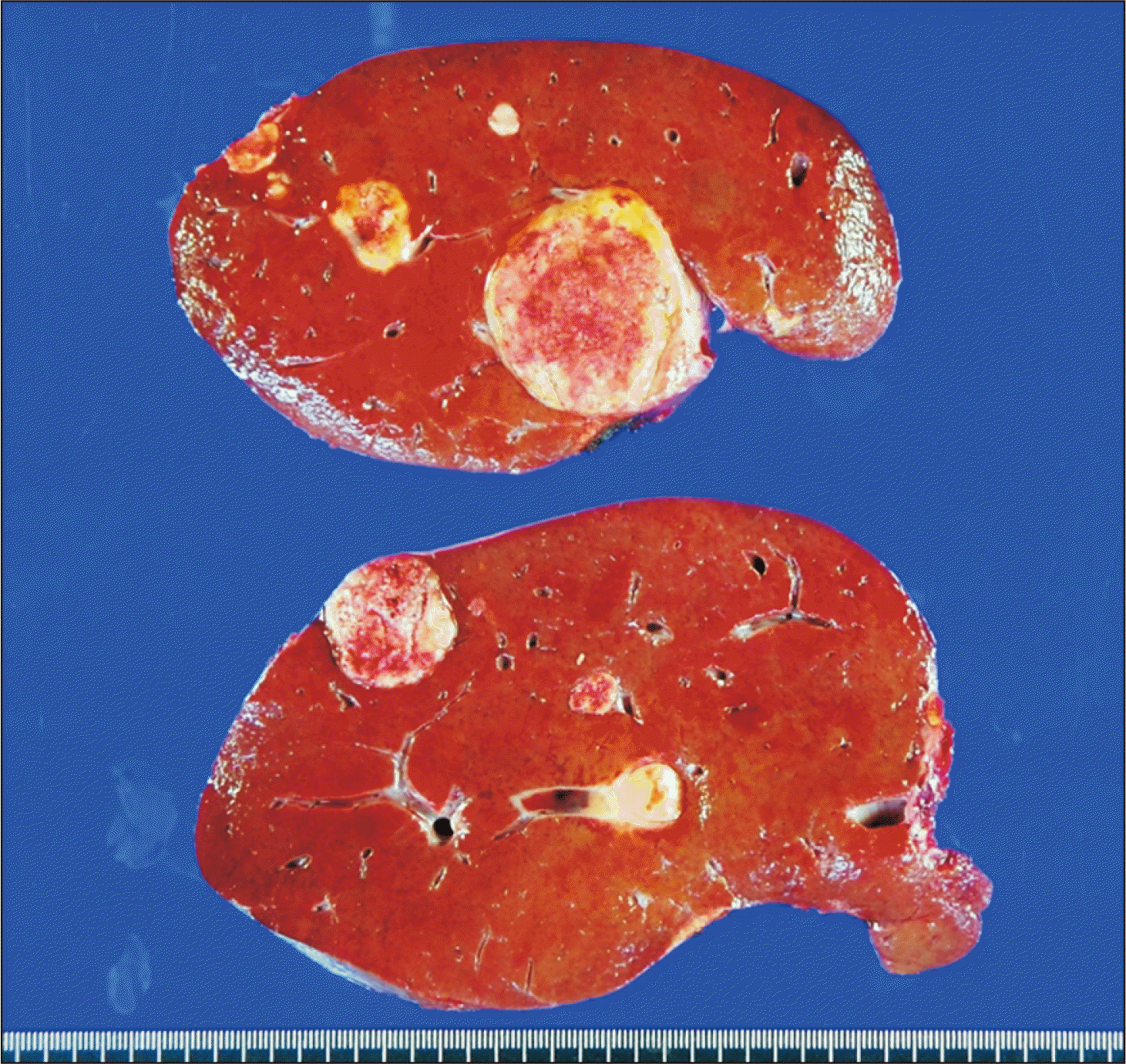
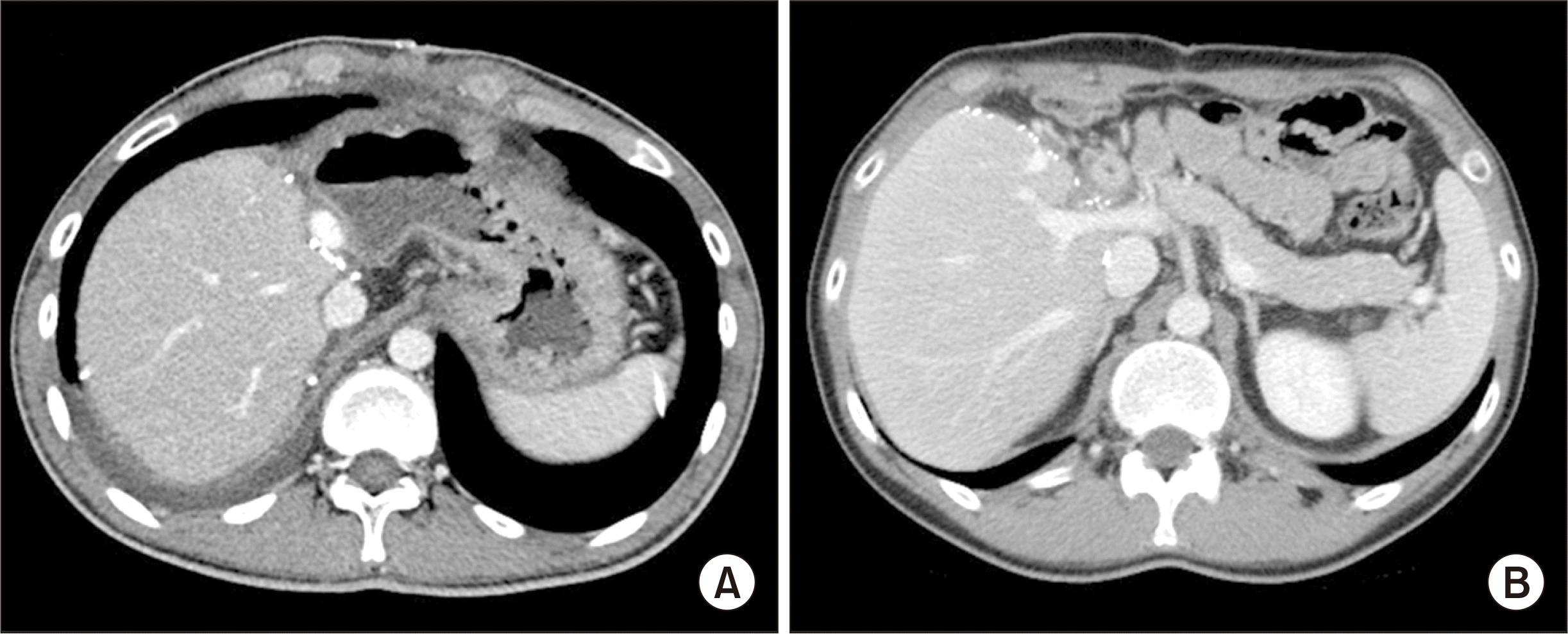
6. Choi JU, Hwang S, Chung IJ, Kang SH, Ahn CS, Moon DB, et al. 2020; Prolonged hepatic inflow occlusion to reduce bleeding during recipient hepatectomy in living donor liver transplantation. Korean J Transplant. 34:55–61.
7. Choi JU, Hwang S, Ahn CS, Moon DB, Ha TY, Kim KH, et al. 2019; Prolonged occlusion of the hepatoduodenal ligament to reduce risk of bleeding and tumor spread during recipient hepatectomy for living donor liver transplantation. Ann Hepatobiliary Pancreat Surg. 23:61–4. DOI:
10.14701/ahbps.2019.23.1.61. PMID:
30863809. PMCID:
PMC6405371.

8. Hwang S, Song GW, Ahn CS, Kim KH, Moon DB, Ha TY, et al. 2020; Nov. 11. Salvage living donor liver transplantation for hepatocellular carcinoma recurrence after hepatectomy: quantitative prediction using ADV score. J Hepatobiliary Pancreat Sci. [Epub].
https://doi.org/ 10.1002/jhbp.863. DOI:
10.1002/jhbp.863. PMID:
33175453.

9. Jabir MA, Hamza HM, Fakhry H, Amira G, Hatano E, Uemoto S. 2017; Anterior versus conventional approach for resection of large right lobe hepatocellular carcinoma. J Gastrointest Cancer. 48:25–30. DOI:
10.1007/s12029-016-9865-x. PMID:
27506210.

10. Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, et al. 2007; Small-sized liver graft does not increase the risk of hepatocellular carcinoma recurrence after living donor liver transplantation. Transplant Proc. 39:1526–9. DOI:
10.1016/j.transproceed.2007.03.066. PMID:
17580180.

11. Liang W, Wu L, Ling X, Schroder PM, Ju W, Wang D, et al. 2012; Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 18:1226–36. DOI:
10.1002/lt.23490. PMID:
22685095.

12. Yoon YI, Lee SG, Moon DB, Ahn CS, Hwang S, Kim KH, et al. 2019; Surgical techniques and long-term outcomes of living-donor liver transplantation with inferior vena cava replacement using atriocaval synthetic interposition graft for Budd-Chiari syndrome. Ann Surg. 269:e43–5. DOI:
10.1097/SLA.0000000000002847. PMID:
30080720.

13. Yoon YI, Lee SG. 2019; Living donor liver transplantation for hepatocellular carcinoma: an Asian perspective. Dig Dis Sci. 64:993–1000. DOI:
10.1007/s10620-019-05551-4. PMID:
30895483.

14. Li H, Yang Z, Tian F. 2019; Clinical characteristics and risk factors for sinistral portal hypertension associated with moderate and severe acute pancreatitis: a seven-year single-center retrospective study. Med Sci Monit. 25:5969–76. DOI:
10.12659/MSM.916192. PMID:
31400275. PMCID:
PMC6699198.

15. Reddy K, Mallett S, Peachey T. 2005; Venovenous bypass in orthotopic liver transplantation: time for a rethink? Liver Transpl. 11:741–9. DOI:
10.1002/lt.20482. PMID:
15973707.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download