Abstract
Common neuropsychiatric complications of tacrolimus include tremors, fatigue, headache, sleep disorders, paranoid reactions, and anxiety. Other, more serious complications include encephalopathy, convulsions, confusion, and coma. To our knowledge, however, severe weight loss by anorexia has not been reported as a neuropsychiatric adverse effect of tacrolimus given to adult kidney transplant recipients. In this article we present two cases of severe anorexia and weight loss associated with tacrolimus that appeared to reverse with cyclosporine.
Go to : 
Neuropsychiatric complications of tacrolimus are typically limited to mild tremors, paresthesia, insomnia, and mood disturbances. More rare are serious reactions of toxic encephalopathy, seizures, and coma [1]. One well-known serious case is that of a renal transplant recipient who suffered progressive neurological deterioration, convulsions, intermittent confusion, and mental obtundation [2]. To our knowledge, however, severe weight loss and anorexia as a neuropsychiatric adverse effect of tacrolimus have not been reported in adult kidney transplant recipients. In this paper, we present two cases.
Go to : 
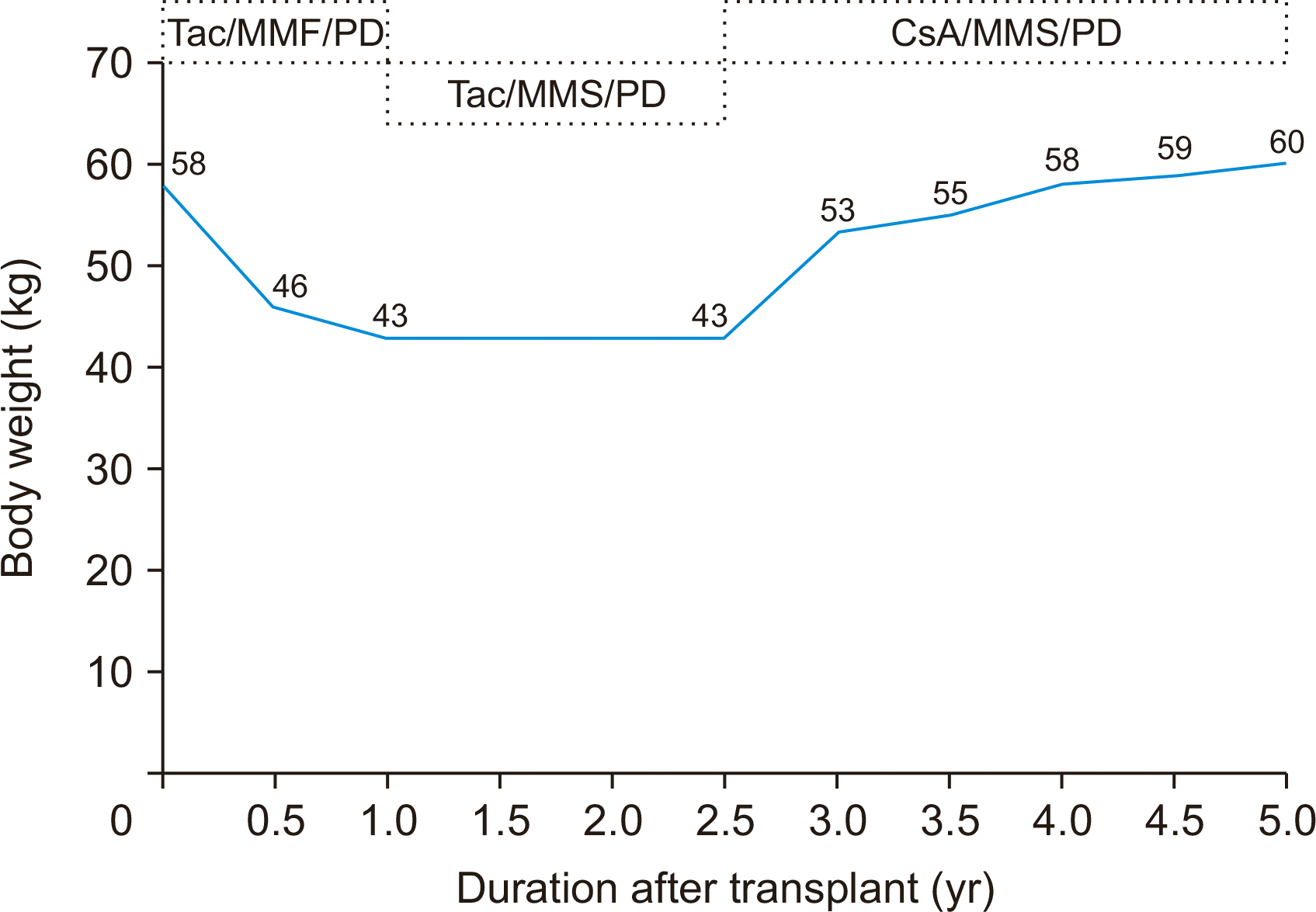
A 57-year-old man (basal weight 58 kg; basal body mass index [BMI], 21.6 kg/m2) was admitted to hospital as a result of losing 15 kg in weight loss (BMI, 16.0 kg/m2) approximately 1 year after kidney transplantation. He also reported watery diarrhea, general weakness, and loss of appetite. Five years ago, he underwent deceased donor kidney transplantation for end-stage renal disease due to chronic glomerulonephritis. His immunosuppressants included tacrolimus, mycophenolate mofetil (MMF), and prednisolone. He received basiliximab induction therapy at the time of transplantation and completed 6 months of trimethoprim-sulfamethoxazole prophylaxis without universal antiviral prophylaxis following the transplantation. No episodes of acute rejection occurred. The patient showed an uneventful course and was in good condition during the early posttransplantation time. On admission his complete blood count was white blood cell count (7.6×103/μL), hemoglobin (12.6 g/dL), and platelet count (353×103/μL). His chemistry profile was total protein (6.9 g/dL), albumin (4.4 g/dL), blood urea nitrogen (23 mg/dL), and creatinine (1.6 mg/dL). Tacrolimus level was 11 ng/mL. Tests for evaluation of weight loss were unremarkable. The results of upper and lower endoscopy showed chronic atrophic gastritis with intestinal metaplasia and nonspecific finding, respectively. We converted from MMF to enteric coated mycophenolate sodium to relieve his symptoms. Conservative treatments, including fluid therapy and nutrient support, resulted in mild clinical improvement. He was admitted intermittently several times afterwards for severe anorexia and cachexia. His serum creatinine levels gradually increased from 1.5 mg/dL to 3.0 mg/dL in the follow-up outpatient period. A graft biopsy was performed and the result indicated minor glomerular change without the finding of calcineurin inhibitor toxicity. Although we have tried to see the relationship of trough level of tacrolimus with anorexia and weight loss, there was no relationship between symptoms and trough level of tacrolimus. Absent any other explanation for anorexia and weight loss, we considered the neuropsychiatric adverse effects of tacrolimus, and switched to cyclosporine.
The cyclosporine level was maintained between 100 and 150 ng/dL. Upon switching to cyclosporine from tacrolimus, the severe anorexia disappeared and he began regaining weight, gaining 10 to 53 kg (BMI, 19.7 kg/m2) over the following 4 months. Five years after his kidney transplant, his most recent weight is 60 kg (BMI, 22.3 kg/m2). He recovered fully and to date his clinical course has been favorable and satisfactory (Fig. 1).
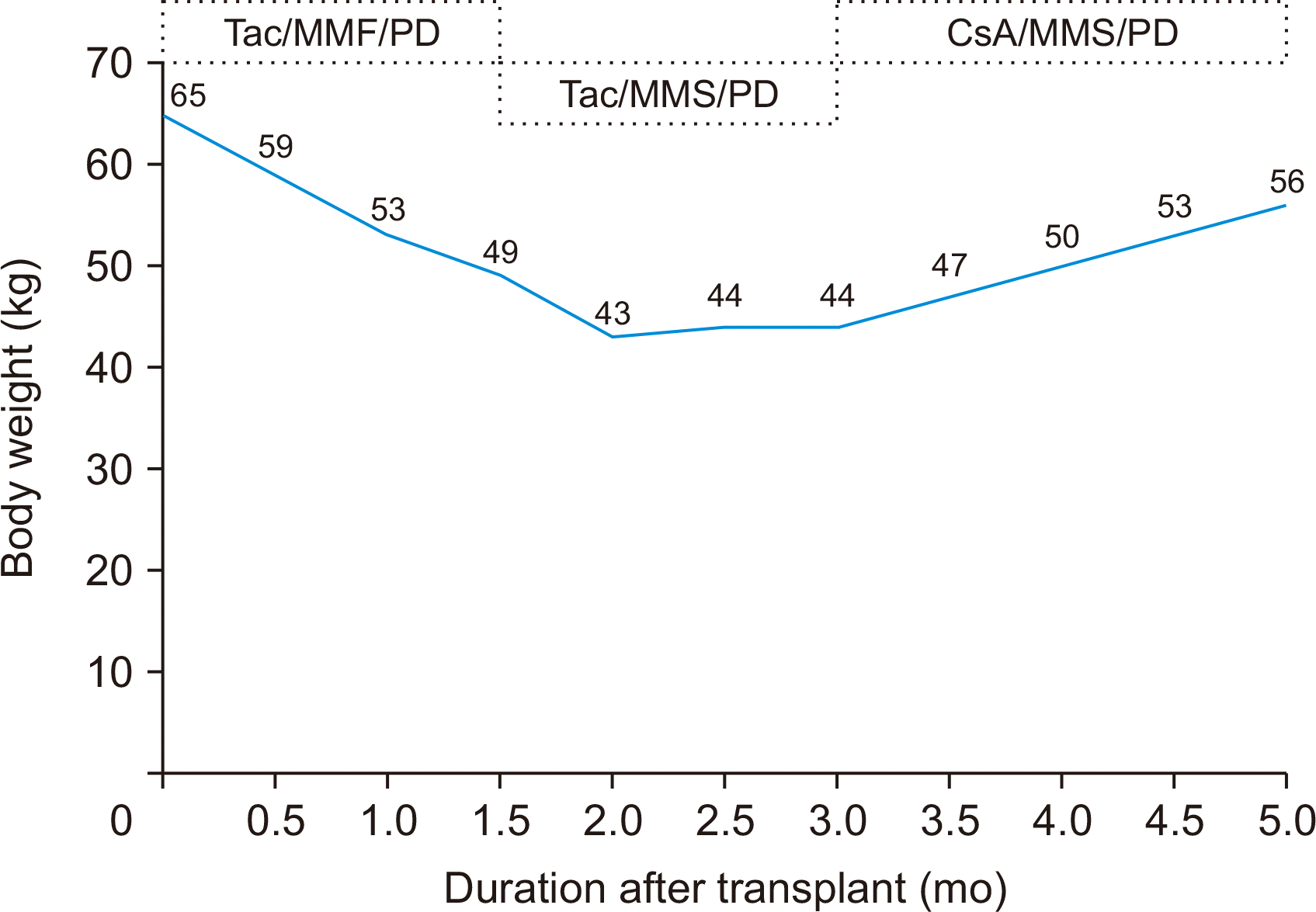
A 55-year-old man visited our hospital with complaints of anorexia and general weakness. His complaints were paired with loose stool and abdominal discomfort. He lost 16 kg weight (BMI, 16.2 kg/m2) from basal weight 65 kg (basal BMI, 21.5 kg/m2) during the approximate 1.5 months after his kidney transplantation. He underwent a deceased donor kidney transplantation with end-stage renal disease due to chronic glomerulonephritis. He received antithymocyte globulin induction therapy at the time of transplantation. His maintenance immunosuppressive regimen consisted of tacrolimus, MMF, and prednisolone. His history showed that he had received his first kidney transplantation from his younger brother 25 years earlier. His maintenance regimen at that time included cyclosporine, MMF, and prednisolone. On admission, his blood count was: white blood cell count (5.8 ×103/μL), hemoglobin (8.5 g/dL), and platelet count (171×103/μL). His chemistry profile was: total protein (5.0 g/dL), albumin (3.1 g/dL), blood urea nitrogen (33 mg/dL), and creatinine (1.2 mg/dL). Tacrolimus level was 9.3 ng/mL. Tests for evaluation of weight loss were unremarkable. Despite conversion from MMF to enteric coated mycophenolate sodium, his symptoms persisted. Even cessation of the enteric coated mycophenolate sodium did not result in an improvement in his condition. Ultimately, recalling our experience in the prior case, we switched from tacrolimus to cyclosporine. The cyclosporine level maintained between 100 and 150 ng/dL. After the switch he showed rapid and progressive improvement as his symptoms of anorexia and weakness abated. Today, his health is good and he continues to gain weight (56 kg; BMI, 18.0 kg/m2) (Fig. 2).
Go to : 
Kidney transplantation is the treatment of choice for end-stage renal disease. For most kidney transplant recipients, a successful kidney transplant improves their quality of life and reduces their mortality risk [3]. Weight gain is more common than weight loss after a kidney transplant. On average, a recipient will gain about 10% in the first year after a kidney transplant [4,5]. A number of factors may be responsible for this weight gain, including the use of immunosuppressive medications and changes in life style (e.g., increased dietary intake and insufficient physical activity) [6].
In this paper we present two cases of severe anorexia and weight loss associated with tacrolimus in adult kidney transplant recipients. To our knowledge, this is the first report to describe very early and late development of severe anorexia and weight loss induced by tacrolimus, and remediation of these effects by switching to cyclosporine. Among past reports, there is one report of a 13-year-old who developed anorexia and weight loss 8 months after starting tacrolimus [7]. Okechuku et al. [8] also showed a case of very early development of anorexia and weight loss induced by tacrolimus after renal transplant that appeared to reverse with cyclosporine.
When unintentional weight loss is observed in kidney transplants recipient it necessitates a diagnostic search that encompasses several possible conditions, just as it does when non-transplanted individuals present with this symptom. There are several potential causes of weight loss, including malignancies, endocrine diseases (e.g., diabetes mellitus, hyperthyroidism, and adrenal insufficiency), infectious diseases (e.g., tuberculosis and human immunodeficiency virus), gastrointestinal disorders (e.g., malabsorption and inflammatory bowel disease), and psychiatric disorders (e.g., anorexia nervosa) [9]. In our cases, diagnostic tests for evaluation of weight loss were unremarkable. Weight loss has also been reported as an adverse effect of MMF but is typically associated with diarrhea and abdominal pain [10]. These symptoms often resolve after dose reduction, by switching to enteric coated mycophenolate sodium or by discontinuation [11]. In our cases, however, weight loss continued even after these measures.
Neuropsychiatric toxicity in kidney patients is usually multifactorial, with patients systemically unwell, uremic and receiving combinations of potentially neurotoxic drugs. Immunosuppressive medications can induce direct or indirect neurotoxicity, which can manifest either in the central or in the peripheral nervous system. However, the exact mechanism of tacrolimus-induced neurotoxicity is not yet well understood. The mechanisms underlying the neurotoxicity of calcineurin inhibitors may be related to up regulation of endothelin receptors A and B and stimulation of endothelin 1 synthesis, damage to the blood–brain barrier, alterations in mitochondrial function, interaction with neuromodulatory systems and vascular toxicity [12,13].
There are some differences regarding the prevalence of clinical side effects between tacrolimus and cyclosporine. Hypertension, hypertrichosis and gingival hyperplasia are less pronounced in the tacrolimus treated patients and an elevated blood glucose level in the cyclosporine treated patients [14]. In addition to these differences, we will need to consider neuropsychiatric difference such as in our cases.
We have tried to see the relationship of the trough level of tacrolimus with patients’ symptoms. However, there was no relationship between symptoms and trough level. The correlation between tacrolimus pharmacokinetics, pharmacodynamics and pharmacogenetics is very complex. Although several factors need to be verified, the understanding of pharmacogenetics may help clinicians to identify high risk patients for neuropsychiatric toxicity [15].
In the two cases described in this paper, the severe anorexia and weight loss resolved with cyclosporine and there were no other apparent causative factors for such. It is, therefore, probable that tacrolimus caused these symptoms. While this is a very interesting observation, a cause-effect relationship cannot be established solely on the basis of this report, and additional studies will be required to confirm this link.
In sum, while there may be several causes of weight loss in kidney transplant recipients, we report two cases of severe anorexia and weight loss induced by tacrolimus. In general, the neuropsychiatric adverse effect of severe anorexia and weight loss rarely manifested in adult kidney transplant recipients. However, once other causes of severe anorexia and weight loss in kidney transplant recipients have been ruled out, clinicians should consider the potential role of tacrolimus. Switching to cyclosporine was the simplest, cheapest and most effective treatment for severe anorexia and weight loss in our patients.
Go to : 
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: SL. Data curation: BHC. Formal analysis: WK. Funding acquisition: SKP. Methodology: KPK. Project administration: HCY. Visualization: SL. Writing–original draft: HPH. Writing–review & editing: SL.
Go to : 
REFERENCES
1. Chegounchi M, Hanna MG, Neild GH. 2006; Progressive neurological disease induced by tacrolimus in a renal transplant recipient: case presentation. BMC Nephrol. 7:7. DOI: 10.1186/1471-2369-7-7. PMID: 16573841. PMCID: PMC1440850.

2. Bechstein WO. 2000; Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 13:313–26. DOI: 10.1111/j.1432-2277.2000.tb01004.x. PMID: 11052266.

3. Suthanthiran M, Strom TB. 1994; Renal transplantation. N Engl J Med. 331:365–76. DOI: 10.1056/NEJM199408113310606. PMID: 7832839.

4. Orazio L, Chapman J, Isbel NM, Campbell KL. 2014; Nutrition care for renal transplant recipients: an evaluation of service delivery and outcomes. J Ren Care. 40:99–106. DOI: 10.1111/jorc.12055. PMID: 24641300.

5. Henggeler CK, Plank LD, Ryan KJ, Gilchrist EL, Casas JM, Lloyd LE, et al. 2018; A randomized controlled trial of an intensive nutrition intervention versus standard nutrition care to avoid excess weight gain after kidney transplantation: the INTENT trial. J Ren Nutr. 28:340–51. DOI: 10.1053/j.jrn.2018.03.001. PMID: 29729825.

6. Aksoy N. 2016; Weight gain after kidney transplant. Exp Clin Transplant. 14(Suppl 3):138–40.
7. Kemper MJ, Spartà G, Laube GF, Miozzari M, Neuhaus TJ. 2003; Neuropsychologic side-effects of tacrolimus in pediatric renal transplantation. Clin Transplant. 17:130–4. DOI: 10.1034/j.1399-0012.2003.00028.x. PMID: 12709079.

8. Okechuku G, Boulos AK, Herman L, Upadhyay K. 2015; Anorexia nervosa in a pediatric renal transplant recipient and its reversal with cyclosporine. Pediatr Transplant. 19:E78–82. DOI: 10.1111/petr.12442. PMID: 25661468.

9. Gupta R, Evans AT. 2020. Approach to the patient with unintentional weight loss [Internet]. UpToDate. Available from: www.uptodate.com. cited 2020 Mar 1.
10. Jacqz-Aigrain E, Khan Shaghaghi E, Baudouin V, Popon M, Zhang D, Maisin A, et al. 2000; Pharmacokinetics and tolerance of mycophenolate mofetil in renal transplant children. Pediatr Nephrol. 14:95–9. DOI: 10.1007/s004670050020. PMID: 10684355.

11. Massari P, Duro-Garcia V, Girón F, Hernández E, Juárez F, Castro C, et al. 2005; Safety assessment of the conversion from mycophenolate mofetil to enteric-coated mycophenolate sodium in stable renal transplant recipients. Transplant Proc. 37:916–9. DOI: 10.1016/j.transproceed.2004.12.020. PMID: 15848574.

12. Sklar EM. 2006; Post-transplant neurotoxicity: what role do calcineurin inhibitors actually play? AJNR Am J Neuroradiol. 27:1602–3.
13. Piotrowski PC, Lutkowska A, Tsibulski A, Karczewski M, Jagodziński PP. 2017; Neurologic complications in kidney transplant recipients. Folia Neuropathol. 55:86–109. DOI: 10.5114/fn.2017.68577. PMID: 28677367.

14. Mihatsch MJ, Kyo M, Morozumi K, Yamaguchi Y, Nickeleit V, Ryffel B. 1998; The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol. 49:356–63.
15. Yu M, Liu M, Zhang W, Ming Y. 2018; Pharmacokinetics, pharmacodynamics and pharmacogenetics of tacrolimus in kidney transplantation. Curr Drug Metab. 19:513–22. DOI: 10.2174/1389200219666180129151948. PMID: 29380698. PMCID: PMC6182932.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download