Abstract
Cancer of the oral cavity and pharynx represents the 7th most diagnosed malignancy in Spain. Mucoepidermoid carcinomas are the most frequent malignancies of the minor salivary glands of oral cavities. The purpose of this report is to describe the very rare case of an alveolar ridge high-grade mucoepidermoid carcinoma presenting as an inside socket radiolucent lesion, simulating an apical cyst. The patient was diagnosed in our unit for oral and maxillofacial surgery and treated with surgery and adjuvant radiotherapy. The patient continues to be free of recurrent/persistent, local/regional disease after two years of follow up. Non-healed tooth related lesions present for more than one year are strongly recommended to be biopsied and evaluated histopathologically.
Go to : 
Cancer of the oral cavity and pharynx is the seventh most diagnosed cancer in Spain with an estimated 8,486 new cases in 20191. Mucoepidermoid carcinomas are the most common malignancies of intraoral minor salivary glands, ranging from 22% to 44% of cases2.
The most frequent location of mucoepidermoid carcinomas is the palate, and can rarely present in the jaw as intraosseous tumors, referred to as central mucoepidermoid carcinomas3.
Due to a tumor’s ability to produce mucin and form cystic spaces, their presentation can resemble ranulas, mucoceles3, or even radiolucent bone lesions2.
Among teeth-associated radiolucent lesions, 73% are classified as apical granulomas, cysts or abscesses and 3% account for malignancies4.
The objective of this report is to present an unusual case of a mucoepidermoid carcinoma masked as a non-healed radiotransparent jaw lesion associated with an endodontic tooth, where clinical findings pointed to a benign condition.
Go to : 
A 54-year-old female was referred to our unit with the chief complaint of one month’s occasional pain from the inferior left first molar and a painless bulge on its gums present for one year. Her relevant past medical history was a successfully treated breast carcinoma (T1cN1bM0) and autoimmune hepatitis.
Intraoral examination revealed a discrete, non-fluctuant, vestibular bulge in the gums adjacent to tooth 3.6, which was previously restored with a crown. Tooth vitality was negative although it was sensitive to percussion.
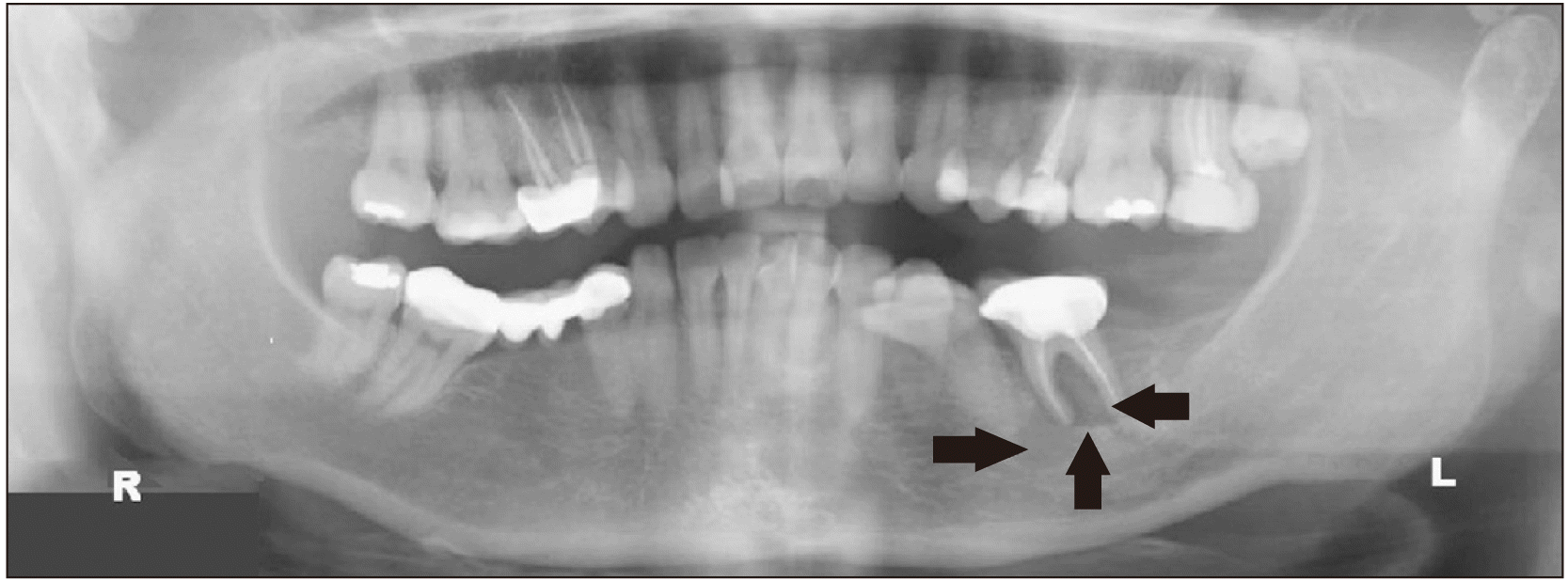
Initial panoramic X-ray showed tooth 3.6 was endodontically treated and presented with a well-defined radiolucent lesion of approximately 15 mm in diameter occupying the socket (Fig. 1), while a 4-year prior X-ray confirmed the lesion was already present but significantly smaller (2 mm). The computed tomography scan revealed a defined soft tissue-density lesion occupying the tooth socket and an external cortical bone defect.
The plan proposed at that time was tooth extraction with curettage of the socket. It was expected to restore the zone with a differed unitary dental implant. During the procedure, the lesion was easily excised, fat-like in consistency, and did not seem to invade the bone.
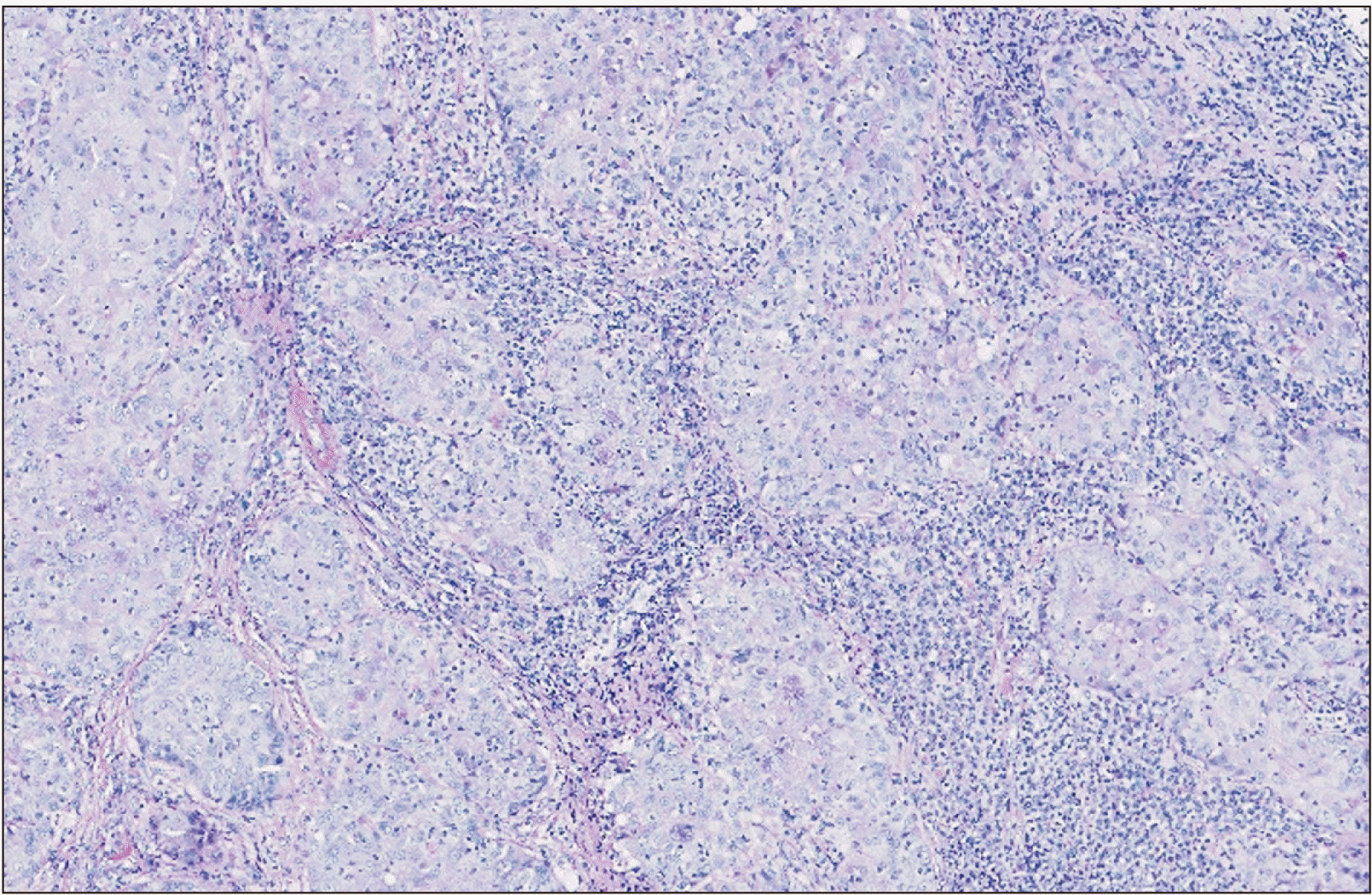
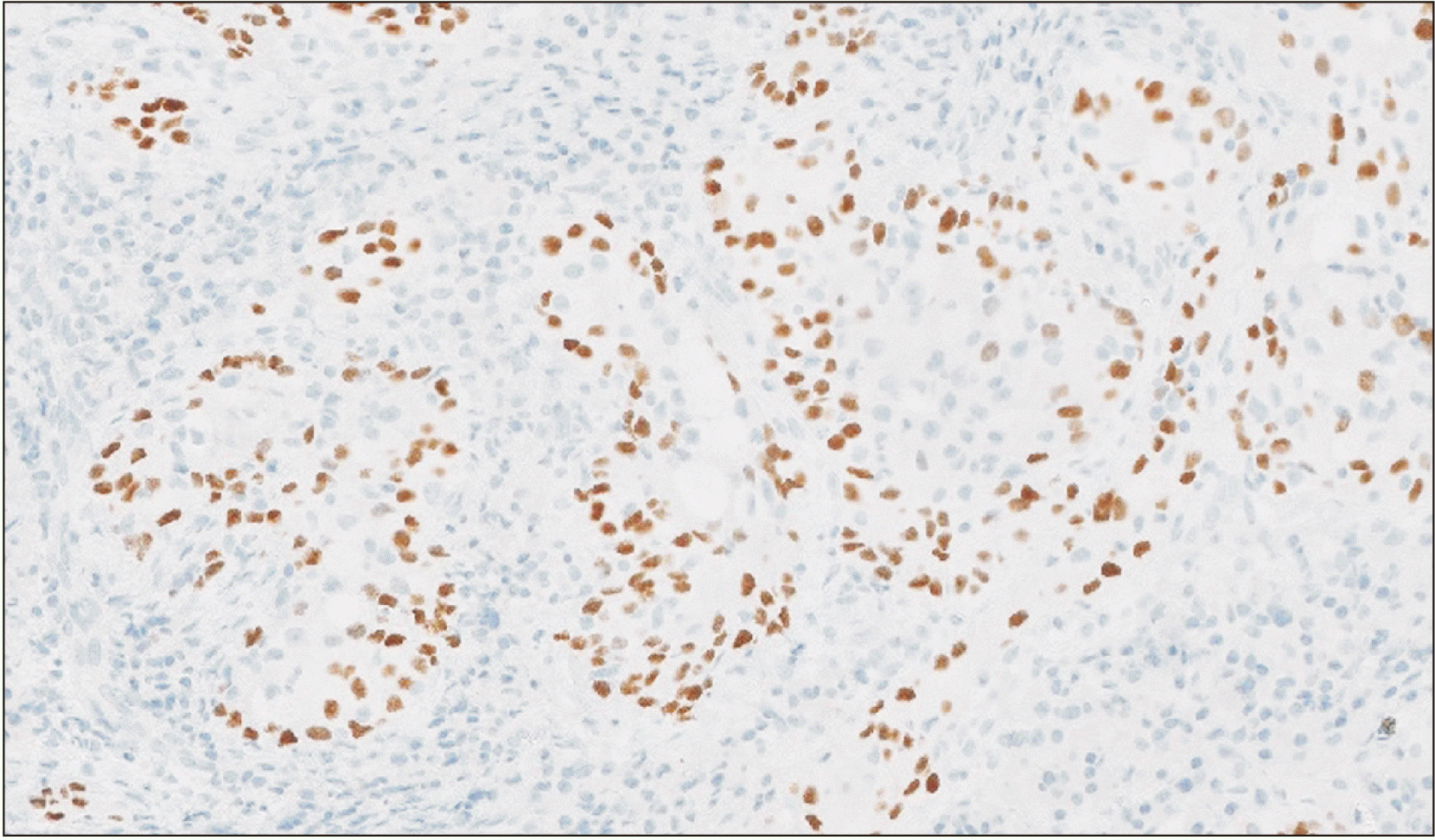
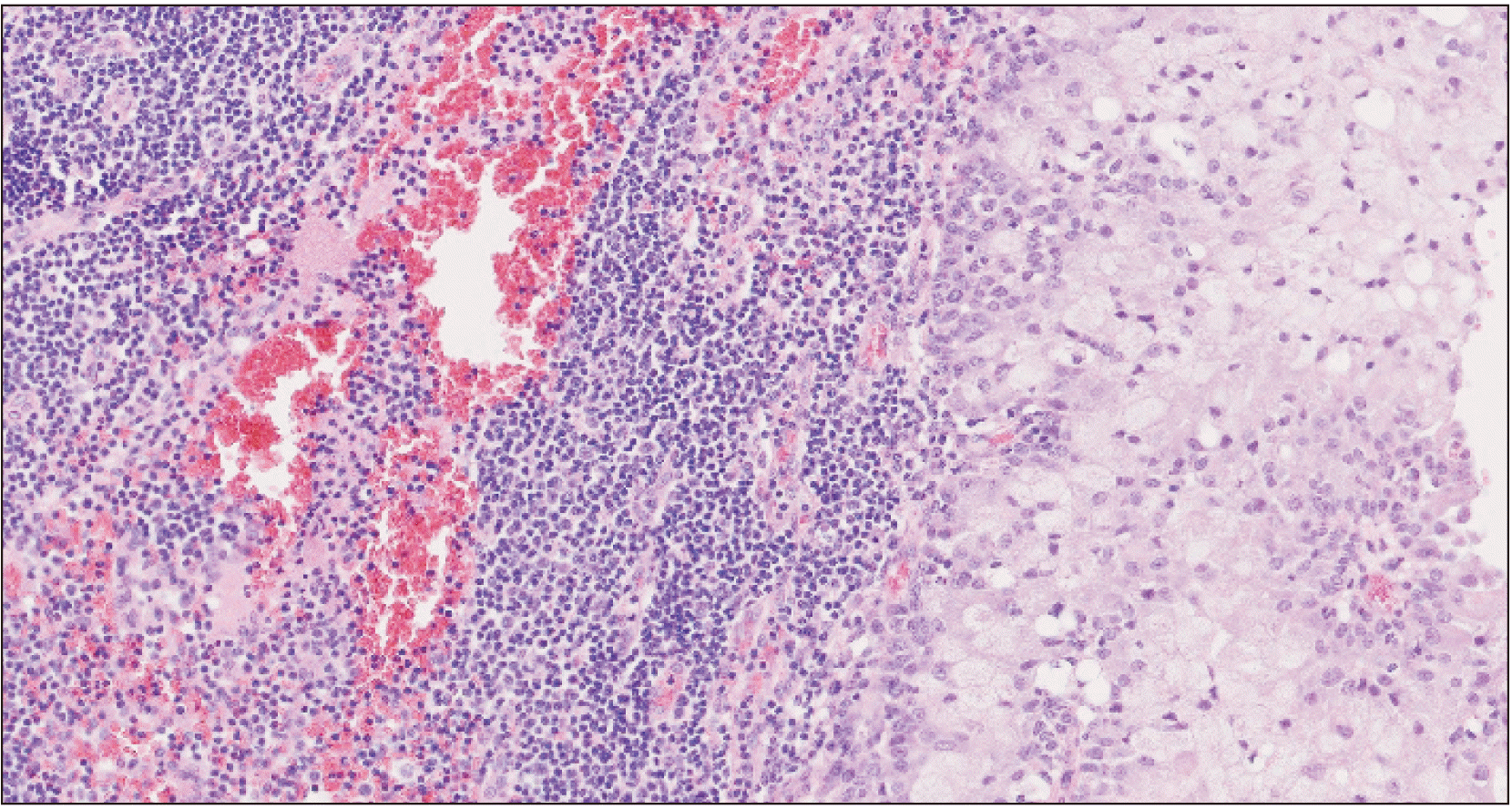
Histopathological evaluation showed a tumor forming solid nests of epidermoid cells principally.(Fig. 2) Given the patient’s oncologic background, immunohistochemical analysis was performed, confirming the tumor’s squamous origin and dismissing mammary origin.(Fig. 3) The findings were compatible with a high-grade mucoepidermoid carcinoma.
This unexpected diagnose required modification of the treatment plan. Physical exploration of the neck did not reveal any existing palpable lymph nodes. A neck magnetic resonance imaging (MRI) and positron emission tomography (PET) scan were urgently scheduled as extension studies. MRI revealed few submandibular left lymph nodes that were not clearly suspicious. PET scan revealed a focal pathologic deposit in the zone containing the healing socket. Macroscopic active distant disease was dismissed.
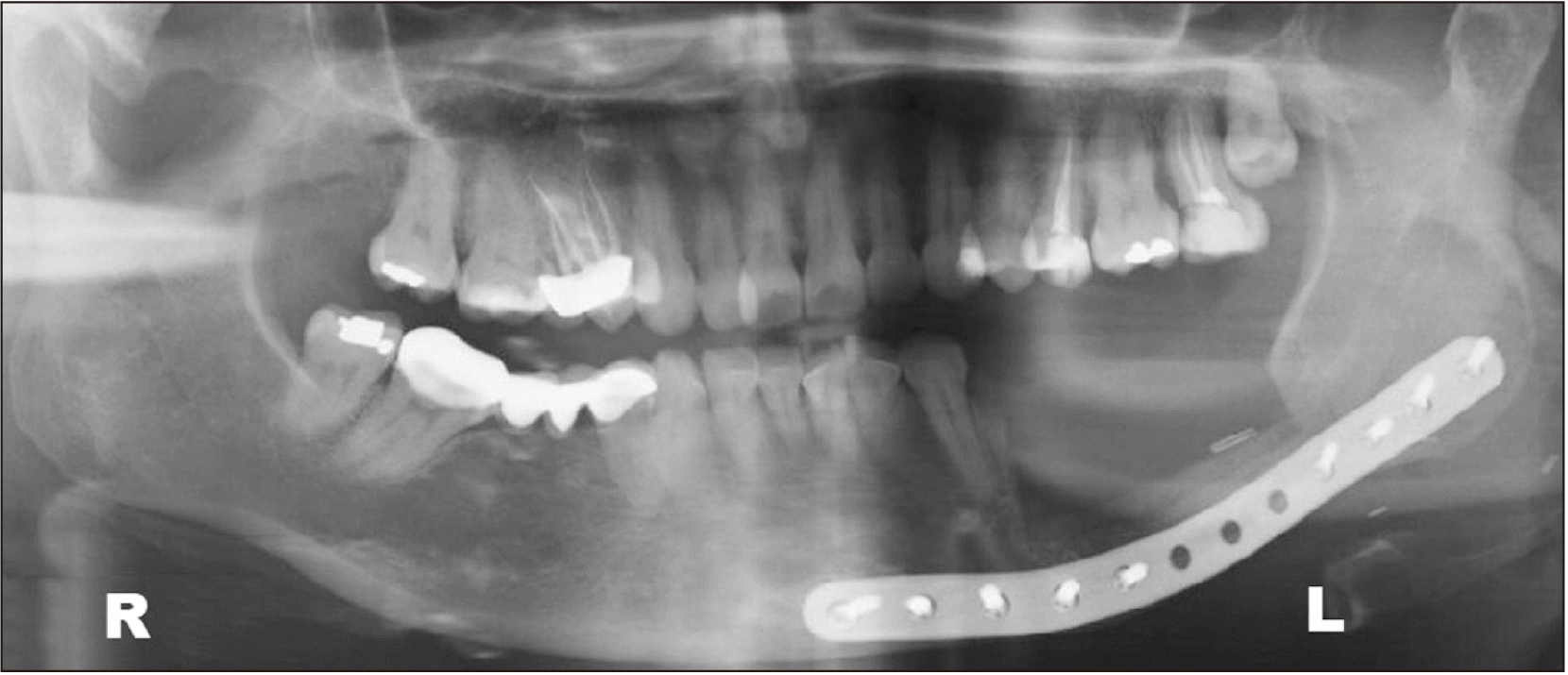
It was explained to the patient that she needed more significant surgery. The resection was outlined in the alveolar ridge soft tissue around the healing socket with a 1 cm margin along with a marginal mandibular body resection. Left supraomohyoid neck dissection was performed. A titanium preformed reconstruction plate (2.0 profile) was placed at the basal mandibular margin to reinforce the segment.(Fig. 4) Intraoral soft tissue defect was achieved using a combination of local vestibular flaps.
Final histopathological analysis revealed an infiltrating high-grade mucoepidermoid carcinoma with 0.4 cm maximum thickness. The tumor was not present within the bone sample. In the neck dissection sample, there was 1/23 intracapsular metastasis.(Fig. 5) No perineural invasion was found.
Final staging was T2N1M0. The patient was referred for evaluation to a medical and radiation oncologist. Adjuvant radiotherapy (intensity-modulated radiation therapy) was conducted two months after surgery, and the total dose for the surgical zone and neck was 64 Gy. The duration of therapy was one and a half months. Correct follow-up was made by both the oncologist and surgical team, and the patient has remained free of new, persistent, or recurrent disease for the last two years.
Go to : 
Most reported mucoepidermoid carcinomas simulated lesions that involved only soft tissues3. Our report is the first where the carcinoma may have emerged from a non-healed apical granuloma, as the main lesion was located inside the tooth socket and directly related to the endodontically treated tooth.
Cystic lesion simulating malignancies can appear as either regular or irregular radiolucent lesions, and can exhibit a mixed pattern of radiolucency, such as with clear cell odontogenic carcinoma5.
The most frequent clinics for mucoepidermoid carcinomas remain asymptomatic, although the most reported symptom was dull pain2. This is consistent with our case, which was only occasionally painful at the end. This behavior should be acknowledged by all clinicians so that non-healed jaw lesions present for more than one year should be biopsied for pathologic evaluation.
Note that PET scan is not helpful in distinguishing benign from malignant lesions3, and therefore the meaning of increased metabolic uptake should be considered carefully, especially when a recent surgery background is present, largely indicating the presence of inflammatory changes in the area.
Tumor grading has prognostic value and is essential for choosing appropriate treatment2. Our report on an infiltrating high-grade tumor treated with surgery and radiotherapy is consistent with achieving good local control; however, this malignancy appears to be radioresistant6.
Expression of Ki-67 antigen is correlated with increased histological grade, as it was found in 10% of high grade tumors. Although immunohystochemical analysis is not performed systematically to the samples; however, we should keep in mind that in high-grade tumors, with the presence of this marker, it is possible to provide additional prognostic information related to the tumor’s biological behaviour6. In our report, immunohistochemical evaluation was helpful to confirm the epidermoid origin of the tumor, clarifying that the tumor was not related with the previous breast tumor.
Although most studies agree that tumor size is the most important predictive factor for survival, both the T stage and N stage are significant survival predictive parameters and are more powerful than the histologic subtype7. Only one study reported that the N stage was not a significant predictor for survival, but it was a predictor for tumor local control8. Perineural invasion was both a negative survival predictor and a treatment failure predictor when a major nerve was involved9.
It is important to relate clinical features (e.g., length of time the tumor has been present) together with both histological features (e.g., pattern of invasion) and surgery-related features (e.g., the presence of positive margins and perineural invasion), as all of these features are directly associated with the clinical course, aggressiveness, and worse prognosis10.
Go to : 
Notes
Authors’ Contributions
D.A.D.M. contributed with case selection and manuscript writing. J.F.P.C. contributed with article’s research. F.M.A. contributed with the case samples’ hystopathologic analysis and provided the case photomicrographies.
Go to : 
References
1. Las cifras del cáncer en España [Internet]. Sociedad Española de Oncología Médica (SEOM);Madrid: Available from: https://seom.org/dmcancer/wp-content/uploads/2019/informe-SEOM-cifras-cancer-2019.pdf. [cited 2019 May 13].
2. Trattner BA, Barak Y, Tordik PA. 2018; Mucoepidermoid carcinoma mimicking a lesion of endodontic origin. J Endod. 44:1303–7. https://doi.org/10.1016/j.joen.2018.04.018. DOI: 10.1016/j.joen.2018.04.018. PMID: 29935872.

3. Sousa Melo SL, Lanzel E, Pagedar NA, Alhazmi D, Dahmoush L, Policeni BA, et al. 2018; Mucoepidermoid carcinoma mimicking a mucocele (ranula) in the floor of the mouth. Dentomaxillofac Radiol. 47:20170331. https://doi.org/10.1259/dmfr.20170331. DOI: 10.1259/dmfr.20170331. PMID: 29231036. PMCID: PMC5991757.

4. Koivisto T, Bowles WR, Rohrer M. 2012; Frequency and distribution of radiolucent jaw lesions: a retrospective analysis of 9,723 cases. J Endod. 38:729–32. https://doi.org/10.1016/j.joen.2012.02.028. DOI: 10.1016/j.joen.2012.02.028. PMID: 22595103.

5. Kim M, Cho E, Kim JY, Kim HS, Nam W. 2014; Clear cell odontogenic carcinoma mimicking a cystic lesion: a case of misdiagnosis. J Korean Assoc Oral Maxillofac Surg. 40:199–203. https://doi.org/10.5125/jkaoms.2014.40.4.199. DOI: 10.5125/jkaoms.2014.40.4.199. PMID: 25247151. PMCID: PMC4170665.

6. Triantafillidou K, Dimitrakopoulos J, Iordanidis F, Koufogiannis D. 2006; Mucoepidermoid carcinoma of minor salivary glands: a clinical study of 16 cases and review of the literature. Oral Dis. 12:364–70. https://doi.org/10.1111/j.1601-0825.2005.01166.x. DOI: 10.1111/j.1601-0825.2005.01166.x. PMID: 16792720.

7. Kakarala K, Bhattacharyya N. 2010; Survival in oral cavity minor salivary gland carcinoma. Otolaryngol Head Neck Surg. 143:122–6. https://doi.org/10.1016/j.otohns.2010.02.033. DOI: 10.1016/j.otohns.2010.02.033. PMID: 20620630.

8. Parsons JT, Mendenhall WM, Stringer SP, Cassisi NJ, Million RR. 1996; Management of minor salivary gland carcinomas. Int J Radiat Oncol Biol Phys. 35:443–54. https://doi.org/10.1016/s0360-3016(96)80005-8. DOI: 10.1016/s0360-3016(96)80005-8. PMID: 8655366.

9. Garden AS, Weber RS, Ang KK, Morrison WH, Matre J, Peters LJ. 1994; Postoperative radiation therapy for malignant tumors of minor salivary glands. Outcome and patterns of failure. Cancer. 73:2563–9. https://doi.org/10.1002/1097-0142(19940515)73:10<2563::aid-cncr2820731018>3.0.co;2-x. DOI: 10.1002/1097-0142(19940515)73:10<2563::aid-cncr2820731018>3.0.co;2-x. PMID: 8174054.

10. Kolokythas A, Connor S, Kimgsoo D, Fernandes RP, Ord RA. 2010; Low-grade mucoepidermoid carcinoma of the intraoral minor salivary glands with cervical metastasis: report of 2 cases and review of the literature. J Oral Maxillofac Surg. 68:1396–9. https://doi.org/10.1016/j.joms.2009.12.019. DOI: 10.1016/j.joms.2009.12.019. PMID: 20363544.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download