This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
Vascular malformation (VM) of the tongue can cause true macroglossia in children. Reduction glossectomy provides primary relief when sclerotherapy has failed or is not possible. In this study, we evaluated the surgical role in functional outcome of reduction glossectomy performed for VM of the tongue.
Patients and Methods We evaluated the functional and surgical outcomes of seven children who were treated at a tertiary care centre in Southern India between 2013 and 2018.
Results
Six children underwent median glossectomy, while one child underwent lateral glossectomy. Functional assessment was performed at least 2 years after the date of surgery. At the time of assessment, speech was comprehensible for three children and was occasionally unintelligible in four children. Taste and swallowing were normal in all seven children. Six children exhibited a minimal residual lesion after surgery, of which only one was symptomatic. Residual lesions were managed with sclerotherapy (n=3), observation (n=2), or repeat surgery (n=1).
Conclusion
Reduction glossectomy in children with macroglossia secondary to VMs has acceptable outcomes in terms of cosmesis and speech, with no gastronomic restriction.
Keywords: Macroglossia, Glossectomy, Vascular malformation, Treatment outcome, Sclerotherapy
I. Introduction
Macroglossia is defined as enlargement of the tongue that results in its protrusion beyond the teeth or alveolar ridge in its natural resting state
1,2. Depending on the extent of involvement, macroglossia has been categorised as generalised or localised
3. Based on aetiology, it is classified as either true or relative macroglossia. In true macroglossia, histological abnormalities are noted in the intrinsic tissue of the tongue that correlate with the findings of tongue enlargement. In relative macroglossia, the histology mostly is non-contributory, and hypoplastic mandible and maxilla proffer a pathological explanation
4. In true macroglossia, when a large protuberant tongue instigates a mass effect, surgery is performed to improve quality of life by reducing the volume of the tongue while preserving function.
Assessment of the functional outcome includes anthropometric measurements of the tongue in paediatric patients undergoing glossectomy based on the normal somatic growth of the tongue. As the size of the tongue varies with age, it is rational to assess the outcome of surgery in terms of function rather than direct measurement of its size. The aim of our study was to evaluate surgical and functional outcomes of reduction glossectomy performed for vascular malformation (VM) of the tongue.
II. Patients and Methods
This is a single-institution, retrospective, descriptive study carried out at a tertiary care centre in India. We included all children with macroglossia due to VM who underwent reduction glossectomy from January 2013 to December 2018 at Christian Medical College, Vellore. This study was performed with approval of Research Institutional Review Board of Christian Medical College, Vellore (IRB No. 13009). Details of the demographic profile, presentation, nutritional status, radiological characterisation, histopathology of the lesion, clinical course, preoperative/adjuvant interventions, all surgical procedures, and presence of residual or recurrent lesions were collated from the medical records. The pathology report and radiological images were reviewed to note the diagnosis and extent of involvement. Nutritional assessment was calculated using World Health Organization (WHO) growth standards. Underweight was considered at less than the 5th percentile weight for age, and stunting was assigned if height was less than the 5th percentile for age according to WHO 2006 and Indian Academy of Paediatrics (IAP) 2015 combined height and weight charts
5.
Outcomes were reviewed after a minimum postoperative interval of two years and entered in a proforma. Variables assessed were feeding habits, taste perception, sensation of the tongue, occlusion, cosmesis, parental satisfaction, and speech output. All variables except speech were assessed by telephonic interviews of one or both parents and the child after obtaining informed consent.
Speech was assessed by understandability. A recorded two-minute audio/video sample of the child’s extempore speech based on a prompt to describe a hobby or school/leisure activity was considered for the assessment. Speech understandability was defined as the degree to which the speaker’s message was understood by the listener. The sample also served to assess the fluency of speech delivery. The recorded samples were assessed by three clinicians for perceptual judgement. One of the three assessors was an independent clinician who was not part of this study. The average score using the Henningsson et al.
6 four-point speech understandability rating scale was employed for final speech assessment. This is a 4-category ordinal scale, with a minimum score of 0 when the speech was within normal limits and constantlyeasy to understand, while a maximum score of 3 denoted speech that was severely impaired and difficult to understand most of the time. A score of 2 or 3 implied difficult to comprehend speech production.
Feeding habits and swallowing were judged with the functional oral intake scale described by Crary et al.
7. This is a seven-point rating score, scored with a minimum of 1 and maximum of 7. A score of 4 or greater indicates oral intake with decreased restriction.
III. Results
During the study period, we treated 43 children with VA of the head and neck region with varying degree of tongue involvement. Seven patients had macroglossia with pure or mixed lymphatic histology that required reduction glossectomy, and all were included in the study. Age at presentation ranged from 1.5 to 5 years (median, 3 years). Outcomes were assessed after 2 to 7.5 years (median interval, 5.16 years) from the date of surgery, which corresponded to the age range of 3.5 to 11 years (median, 8.6 years).
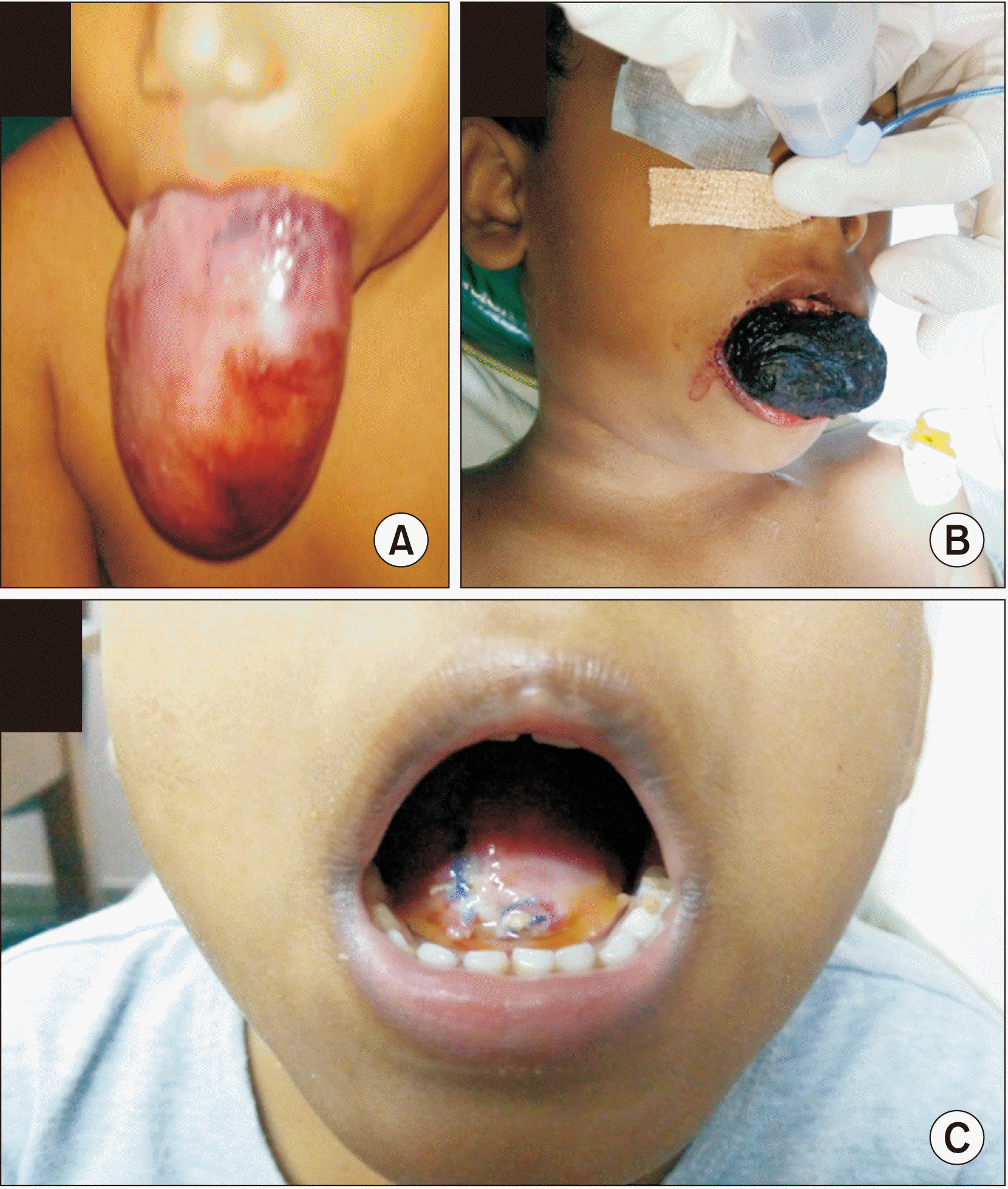
Six of the seven children had the tongue lesion at birth, the other presented at 11 months of age. Progression of disease was gradual in five patients, while two patients exhibited a sudden increase in tongue size. At time of presentation, all patients had grossly enlarged and protruded tongue with inability to appose their lips.(
Fig. 1. A, 1. B) None of the patients had stridor, sleep apnoea, or features of micro aspiration or nutritional anaemia. Despite the grossly enlarged tongue, all seven patients were able to ingest liquids orally. Other symptoms at presentation are depicted in
Table 1.
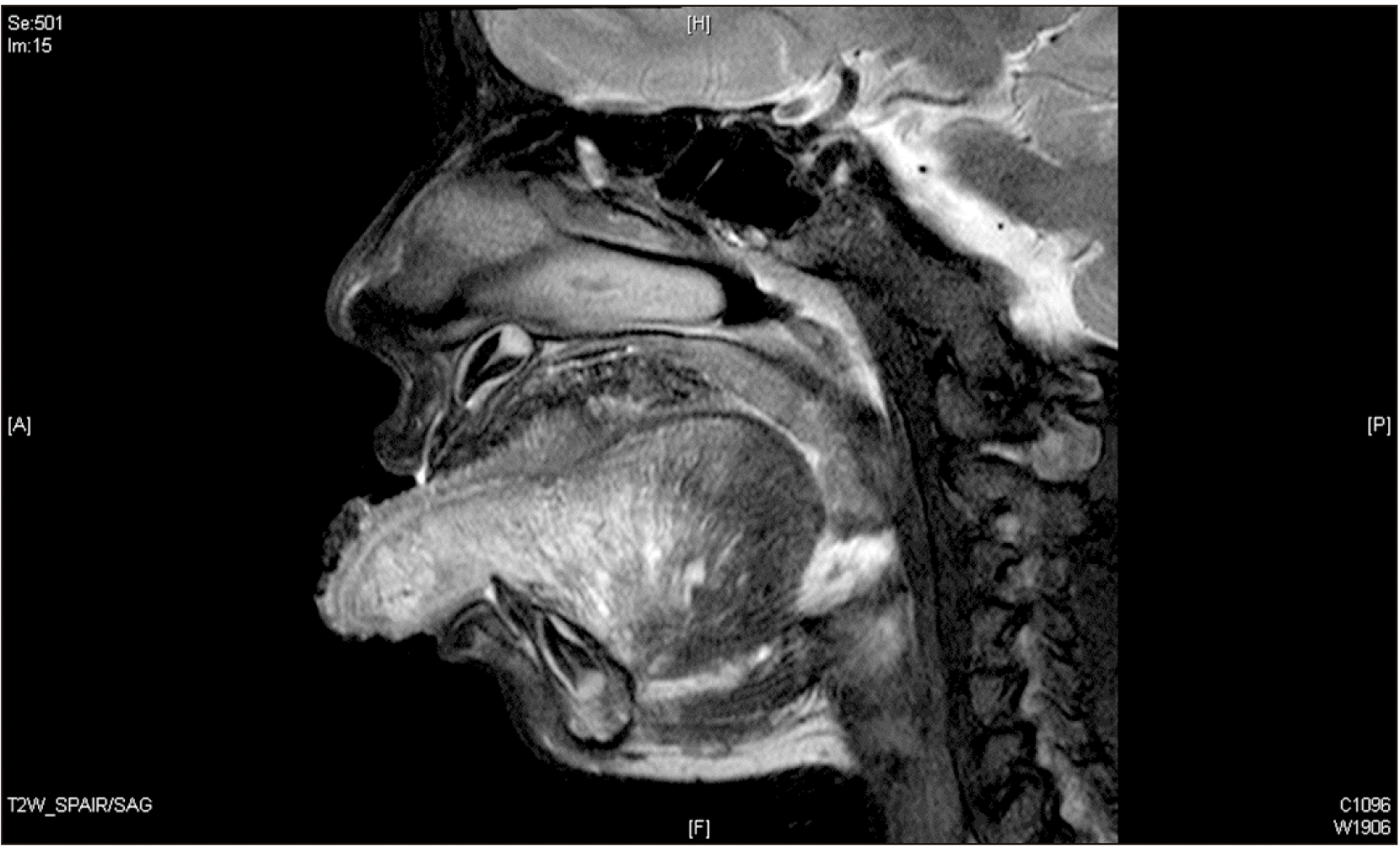
Magnetic resonance imaging was performed for all patients to assess the extent of tongue involvement.(
Table 1,
Fig. 2) In one patient, the lesion was lateralised to the left side, five showed varying degrees of diffuse involvement of the tongue, and one lesion predominantly involved in the anterior two-thirds of the tongue. All lesions were slow flow lesions and were either lymphatic, venous, or mixed veno-lymphatic. One patient exhibited intrathoracic extension.
Reduction glossectomy was performed in all patients. Five of these children underwent preoperative sclerotherapy either with sodium tetradecyl sulphate foam or bleomycin foam, and three patients exhibited some form of a response. One patient underwent marsupialization of the tongue lesion before visiting our institute with no effect. The indication for reduction glossectomy was primarily size of the tongue or intervening complications like bleeding, necrosis, or recurrence. The surgical procedures performed were Köle inverted V glossectomy (n=6) and unilateral hemi glossectomy (n=1).
Two patients with extensive neck involvement exhibited a compromised pharyngeal space and airway after sclerotherapy. Both patients had to undergo debulking of the neck lesion with tracheostomy. One child required an adjuvant short-term feeding gastrostomy.
There were no surgical complications from glossectomy per se, but there was bleeding from the tracheostomy in the two such treated children.
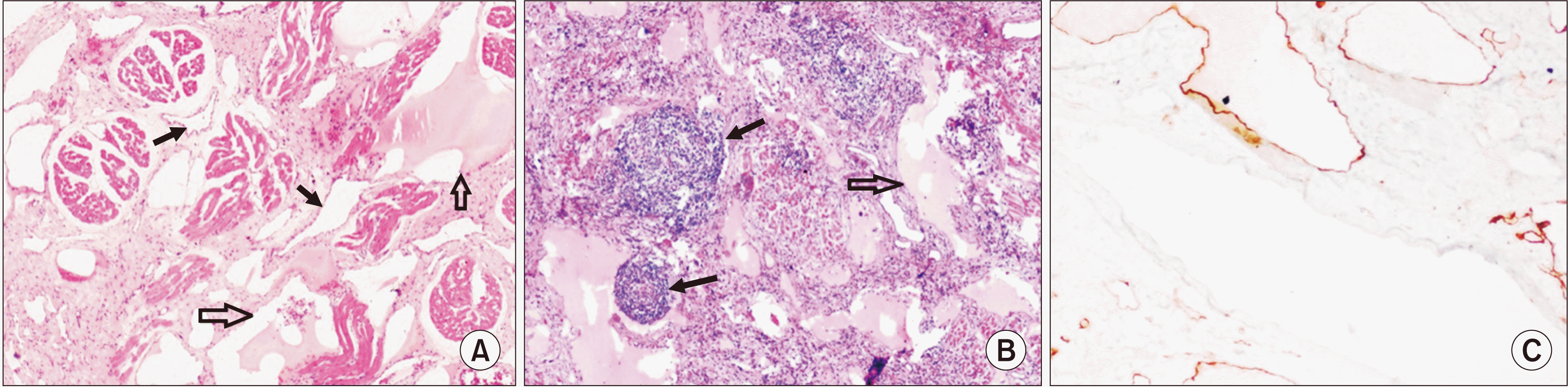
Pathological examination revealed lympho-venous malformation (n=4) and lymphatic malformation (n=3). These vascular lesions involved the subepithelial region, penetrating between the skeletal muscle fibres and extending into the deep underlying adipose tissue.(
Fig. 3)
Six patients had residual disease in the tongue, floor of the mouth, or neck. All tongue lesions were small except one that became prominent episodically with every upper respiratory infection. The residual lesions were managed with revision glossectomy in one child, observation in two children, and adjuvant sclerotherapy (n=3) in three children. Only two patients responded completely to adjuvant therapy, while the others remain asymptomatic with small residual lesion.
Assessment of the outcomes of surgery showed that all patients could accommodate their tongues within their dental arches at rest. Speech was assessed in all seven patients and was comprehensible in all of them, with a score of 0 (n=3) or 1 (n=4). All patients exhibited good initiation of conversation without any social restriction or frustration about their articulation.
All children exhibited some degree of residual malocclusion, but only one child had difficulty biting with their incisors due to reverse overjet. All children reported thermal sensations and were able to distinguish between saltiness, sweetness, and bitter tastes. After surgery, all patients exhibited improvements in feed tolerance to a score of 7. On nutritional follow-up, all children had significant improvement in nutritional status in terms of improvement in percentile, in accordance with the chart. All also showed significant improvement in nutritional status in terms of height and weight.(
Tables 2,
3) The parental and patient satisfaction with the surgical outcome was universal in our cohort.
IV. Discussion
Vascular anomaly (VA) is classified as vascular tumour or VM
8. Pure lymphatic malformation or combined lymphovenous malformation is the most common cause of macroglossia in children and is apparent at birth in 60% of all such patients, with 95% becoming symptomatic after 2 years
2. Our cohort showed similar findings. Management of VA depends on type of lesion, area of involvement, type of presentation, and associated symptoms
9-11. Sirolimus is a novel adjuvant therapy for VM with promising reduction
12-15. It is not available for over the counter use for this condition despite being efficacious because of major blood dyscrasias and gastrointestinal toxicities in a significant proportion of users
12. Patients must be monitored for recurrence or long-term toxicities, and sirolimus use should be sanctioned by a constituted medical board. This cohort was not exposed to sirolimus as the patients had advanced disease at presentation.
Reduction glossectomy procedures are subdivided into two groups: glossectomy along the median line and peripheral glossectomy
9. Care in preserving the lingual arteries and hypoglossal nerve ensures maintenance of the viability and function of the tongue. Since the neurovascular bundle lies ventrally on either side of the tongue, a median approach greatly reduces the chance of injury.
The most widely practiced reductive procedures for true macroglossia are keyhole techniques described by Morgan et al.
16 and resection and approximation of the tongue by Köle
17-
21. The keyhole technique is preferred to reduce the central bulk of the tongue. In our study group, six patients with diffuse involvement of the tongue underwent the Köle procedure, while one child with localised lesion underwent hemi glossectomy. All patients had acceptable outcomes with good cosmesis, satisfactory speech understandability, decent taste perception, and normal swallowing. Therefore, Köle’s procedure is appropriate for diffuse involvement as it is technically easy to perform in a tongue of massive proportion.
Speech is the efficient primary mode of human communication and is essential for emotional well-being. Speech outcomes after glossectomy have been extensively studied in the adult population using the Speech Handicap Index (SHI). Adult studies demonstrated that SHI will improve with time and return to its baseline preoperative score after approximately 1 year of surgery, but with persistent clinical impairment
22. Paediatric SHI validated for Indian languages are unavailable. Therefore, we used the speech understandability scoring developed by Henningsson et al.
6. In all patients, speech quality improved to a score of 0 (n=3) or 1 (n=4) after surgery. Four of the six children who underwent Köle’s procedure exhibited a score of 1, but all the other children including the patient who underwent hemi glossectomy had normal speech with a score of 0. Abnormalities observed in three children with a score of 1 wereoccasional omission and distortion. Good initiation of speech without any frustration about the quality of articulation or social restriction implies that there is no detectable emotional distress due to articulation.
The mechanical role of the tongue is essential for bolus transfer in mastication and oropharyngeal transfer of the bolus while initiating deglutition. After surgery, all patients achieved near normal chewing and swallowing, which was evident by improvement of their functional oral intake score and nutritional status. It has been demonstrated in adults, following partial or total glossectomy, that most patients develop more than one compensatory mechanism to overcome swallowing-related impairments and complications
23. In all patients, we observed nutritional recovery with improvement in growth percentile.(
Tables 2,
3)
The localisation of taste to distinct areas of the tongue became obsolete with these patients, and taste was appreciated on the palate, epiglottis, and proximal oesophagus. Taste buds are concentrated around the circumvallate papillae, and a moderate number of them are found on the anterior and lateral surfaces of the tongue
24,25.In Köle’s procedure, there is loss of tissue from the anterior aspect and tip of the tongue. In all patients, we aimed to reduce loss of tissue near the circumvallate papillae. This would preserve more taste buds and conserve greater taste sensation.
Reduction glossectomy was carried out to reduce tongue bulk with preservation of adequate tissue. After glossectomy, six patients had minimal residual lesions on the tongue. With adjuvant therapies, all except one patient continued to be asymptomatic without an increase in size of the lesions. The symptomatic child underwent revision surgery. Aggressive excision of the tissue might impair function. As most residual lesions are asymptomatic, it is worthwhile to compromise the completeness of resection to leave adequate tongue tissue for a better functional outcome. Residual lymphatic malformations, which are exacerbated with upper respiratory tract infections, can be managed with antibiotics
19. Larger lesions on the tongue and neck can be addressed with sclerotherapy with or without re-excision or debulking.
Speech was assessed by three assessors, of which two were part of the treating team. This could have created an observational bias. However, the scores given independently by all three assessors were comparable. Other limitations of the study were the small sample size, wide range in age of children, and retrospective nature of the study. Despite these limitations, the study remains relevant, as glossectomy in the paediatric age group is a rare surgical procedure, and adequate literature is not available for functional outcome after glossectomy in children with VM.
This series demonstrates that functional outcomes of speech, gustatory appreciation, oral appreciation of thermal sensory input, and deglutition are largely preserved with proper surgical technique. This is congruent with other studies where reduction glossectomy was performed for macroglossia due to other non-malignant pathologies
18,26,27.
V. Conclusion
Reduction glossectomy can be considered a reliable option to control macroglossia caused by lymphatic and veno-lymphatic malformations after failed non-surgical therapy. Symptomatic residual lesions on the tongue following glossectomy can be managed conservatively in most instances.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download