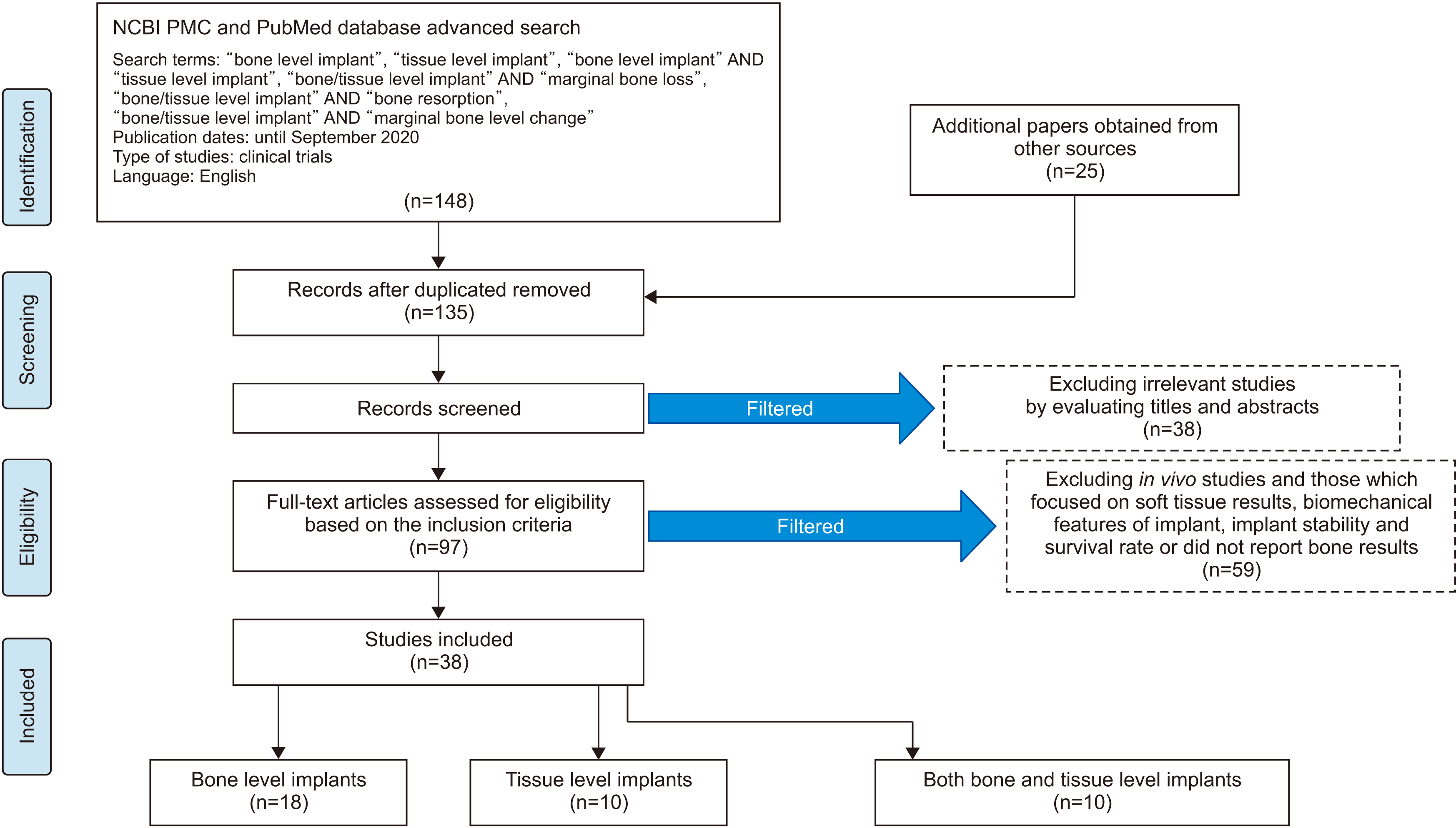
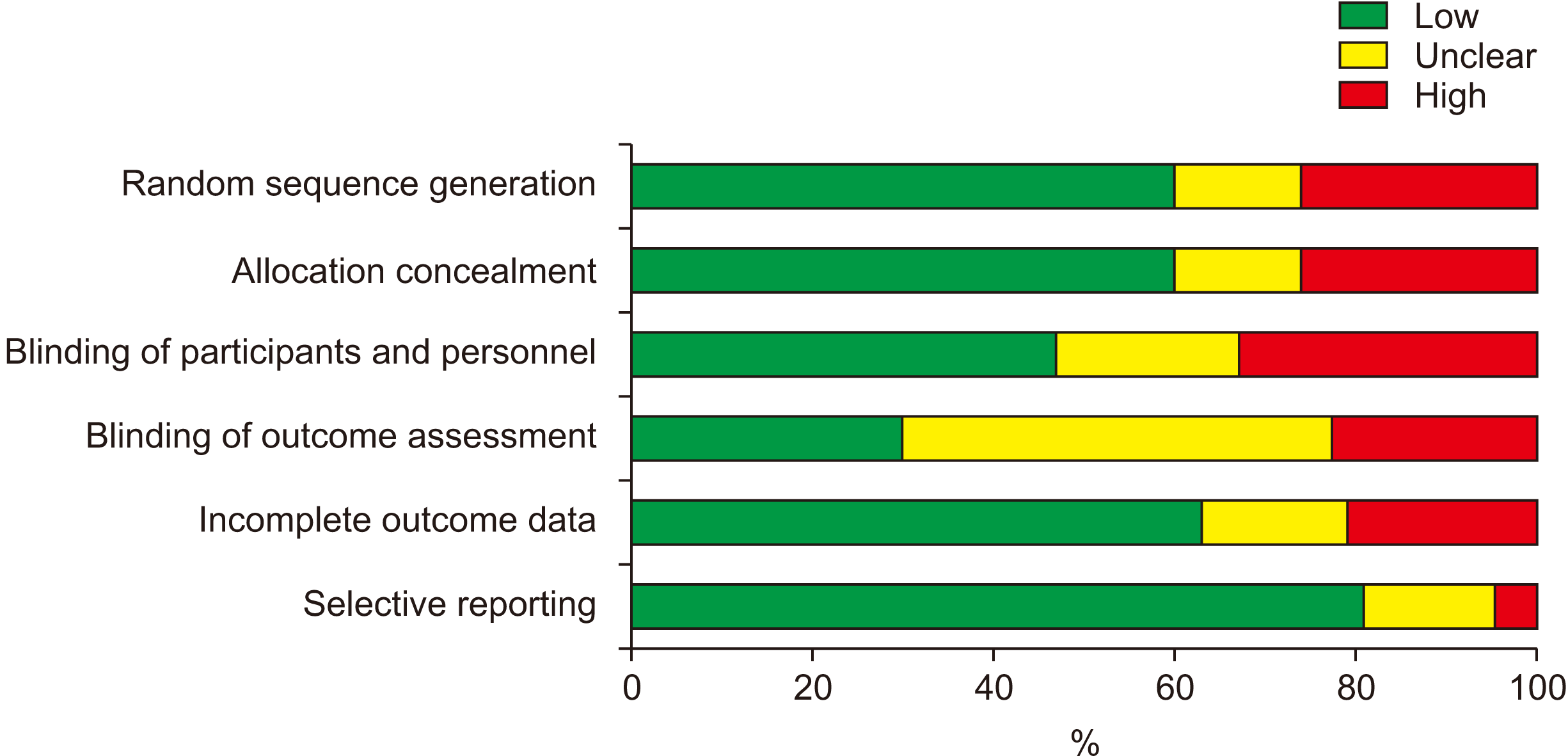
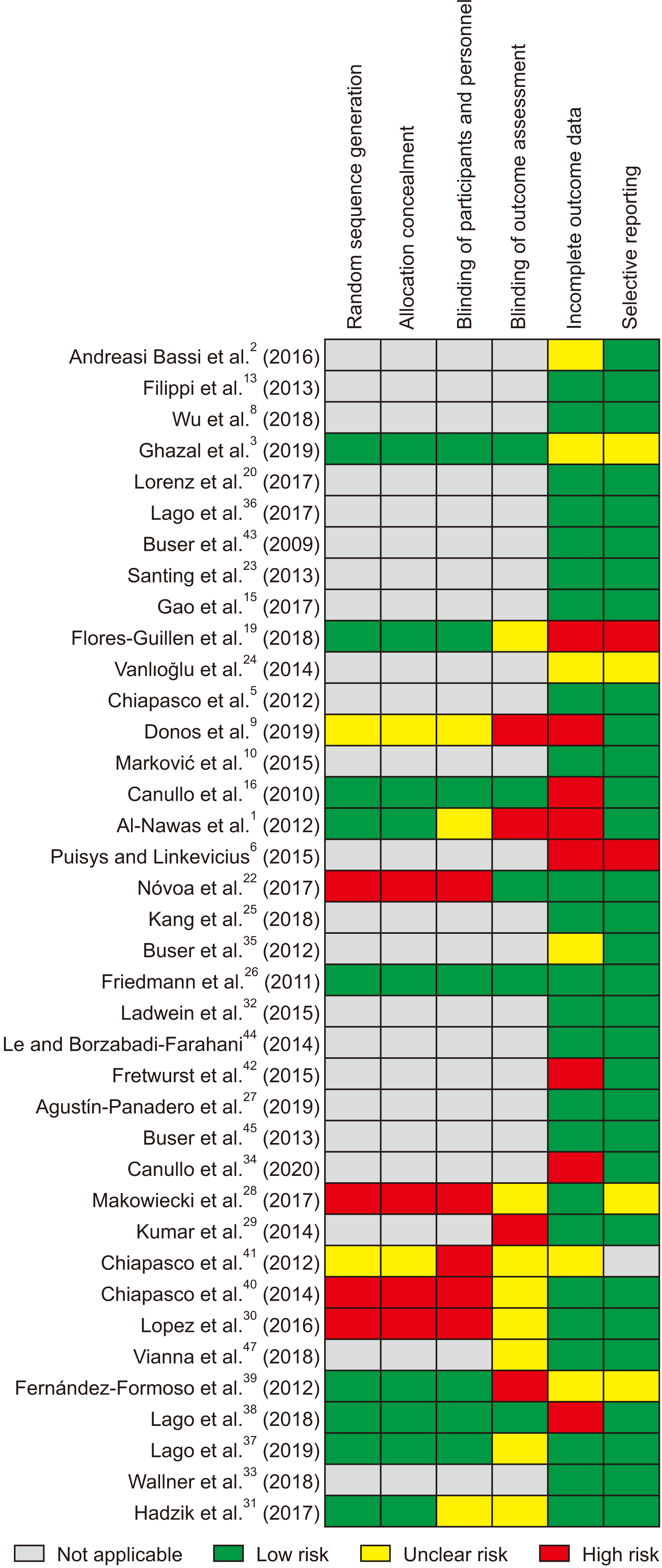
Abstract
Notes
References
Table 1
| Study |
Study type |
No. of implants |
Study design |
Implant placement area | Bone results | Other results | Measurement device |
Sex & age (yr) |
Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Andreasi Bassi et al.2 (2016) | Prospective | 52 |
Cylindrical & tapered Internal hexagonal Bone graft |
Max/Man Ant/Post |
Mean MBL: 77% Mean peri-implant bone loss: 0 mm |
SVR: 100% Worse results only in implant supporting bridges. |
Panoramic PA |
21 F/31 M Mean age: 54 |
44.6 mo (mean) |
| Filippi et al.13 (2013) | Prospective multicenter non-interventional | 908 | SLActive |
Max/Man Ant/Post |
No change in crestal BL (62.2% & 61.8% mesial & distal) BL change >1 mm in <5% of implants (4.5% & 3.9% mesial & distal) Bone growth around 3% of cases (3.3% & 3% mesial & distal) |
SVR: 98.5% SCR: 96% |
PA |
852 (55.6% F/44.2% M) Mean age: 53.7 |
1, 2 & 3 yr |
| Wu et al.8 (2018) | Retrospective | 114 |
Internal hexagonal Fresh socket GBR (30 patients) A: submerged B: nonsubmerged |
Max/Man Ant/Post |
No differences in MBL Mean MBL:1st yr (majority of MBL): A: 1.20 mm B: 1.17 mm5th yr: A: 1.98 mm B: 1.94 mm Mean MBL <1.5 mm within the 1st yr Mean MBL between 1 & 5 yr:A: 0.78 mm B: 0.77 mm |
SVR:A: 94%B: 96% No difference in failure time. Implants with a diameter of 3.0 mm: lower SVR |
CBCT Panoramic PA |
42 M/30 F 55 patients <60 yr, 17 patients >60 yr |
Annually for 5 yr |
| Ghazal et al.3 (2019) | Prospective RCT | 47 |
TiZr SLActive T: narrow (3.3 mm) C: standard diameter (4.1 mm) |
Max/Man Ant/premolar |
Mean BL change:6 mo: similar12 mo: T: –0.27 mm C: –0.48 mm |
SCR & SVR: 100% No differences in gingival recession & patient satisfaction. |
PA |
50 (M 36%/F 64%) Mean age: 51.2 |
6 & 12 mo Postloading |
| Lorenz et al.20 (2017) | Retrospective follow-up | 47 | GBR (synthetic bone graft: HA & b-TCP) | Max/Man | Mean bone loss: 0.55 mm(range, 0-3 mm) |
Low median rates for PD (2.7 mm) & BOP (30%) Mean PES: 10.1 from 14. |
Radiological |
11 F/9 M Mean age: 58.5 |
36-48 mo Postloading Mean: 42.6 mo |
| Lago et al.36 (2017) | Prospective clinical | 67 | Platform switched |
Max/Man Post (premolar) |
Mean MBL: Baseline to 1 yr: 0.06 mm1 to 5 yr: 0.23 mmBaseline to 5 yr: 0.28 mm No difference from baseline to 1 yr. BIC:Baseline: 0.64 mm1 yr: 0.59 mm5 yr: 0.35 mm |
Soft tissue: Papilla tip to contact point distance: Baseline: 2.08 mm 1 yr: 1.54 mm 5 yr: 1.31 mm Difference between 1 & 5 yr & baseline & 5 yr No difference in buccal margin. |
PA |
20 M/15 F Mean: 47.1 |
1 & 5 yr |
| Buser et al.43 (2009) | Prospective case series | 20 |
Sand-blasted & acid-etched surface GBR |
Max Ant (single tooth) |
12 mo: Mean bone loss: 0.18 mm (1 implant with bone loss >0.5 mm & 15 with minimal bone loss [< 0.25 mm]) Mean DIB:3 mo: 0.09 mm 6 mo: 0.14 mm12 mo: 0.18 mm |
12 mo: Mean: PI: 0.36 SBI: 0.26 PD: 4.43 mm Facial DIM: –3.53 mm No severe recession (1 mm) |
PA |
5 M/15 F Mean age: 41.7 |
1, 3, 6, 12 mo |
| Santing et al.23 (2013) | Prospective cohort | 60 |
Platform-switched A: augmented B: non-augmented |
Max Ant (single tooth) |
18 mo: Mean BL change: –0.10 mm No differences between A & B. |
Mean PD: 2.57 mm ICAI & PES: Less favorable in A. |
NM |
60 >18 yr |
7 & 18 mo |
| Gao et al.15 (2017) | Open-label single-arm observational | 22 | Platform switched & SLActive +autogenous bone |
Max Ant (single tooth) (tooth position: 14-24) |
MBL:Most implants (95.5%) <0.5 mm & one (4.5%) with 2.12 mm change from baseline to 36 mo (mean, 0.07 mo) Crestal BL decreased, from 2.34 mm at baseline to 1.70 mm at 36 mo. |
SCR: 100% SVR: 100% Functional SCR: 95.5% Increase in mean stability from provisional & final prosthesis. Facial gingival margin & papilla: stable Mean PD: 12 mo: 1.26 mm 24 mo: 1.02 mm 36 mo: 1.40 mm |
PA |
8 M/13 F Mean: 44.97 |
6, 12, 24, 36 mo |
| Flores-Guillen et al.19 (2018) | Prospective RCT | 30 |
SLActive Platform switched A: Submerged B: Transmucosal +GBR |
Max Ant Non-molar sites (single tooth) |
Mean bone loss:A: 0.59 mm B: 0.78 mm Similar bone gain after 1 yr. 5 yr: Bone loss of <1 mm in 76.7% of implants. A: greater bone loss for 1 yr (0.73 mm) than 1-5 yr (–0.10 mm) B: 0.73 mm (1 yr) & 0.04 mm (1-5 yr) |
SVR: 100% Peri-implantitis (crestal BL ≥2 mm) & BOP only in one patient. Similar responses with significant increase in PI in mesial & distal papillae. |
PA |
23 M (57.5%) 17 F (42.5%) Mean age: 48.5 |
1, 2, 3, 4, 5 yr |
| Vanlıoğlu et al.24 (2014) | Clinical trial | 55 | Platform switched |
Max Ant (single tooth) (central to premolar) |
Successful integration of all. MBL <0.5 mm for all. Mean bone loss at final recall: 0.12 mm (No significant difference) No difference in MBL in Zr & Ti abutments. 7 implants with increase in level of bone contact. MBL: Baseline: 0.08 mm 1 yr: 0.1 mm 2, 3, 4 yr: 0.12 mm |
SVR: 100% Complete papilla fill in 89.09% Mean PD: Baseline: 2.1 mm 2, 3 yr: 2.2 mm 4 yr: 1.9 mm |
Panoramic PA |
23 F/24 M Mean: 33 |
2, 3, 4 yr |
| Chiapasco et al.5 (2012) | Clinical trial | 60 |
Platform switched Autogenous cortical grafts from ramus or calvarium |
Max/Man(post & partial edentulous vertical & horizontal defects) |
Mean bone loss before implantation: 0.18 mm (calvarial grafts) 0.42 mm (ramus grafts) Mean MBL after implantation: 0.41 mm (calvarial grafts; mostly 0 mm) 0.52 mm (ramus grafts; mostly 0-1 mm) |
SVR:100% for both groups SCR:90.3% in calvarial graft93.1% in ramus grafts |
Panoramic PA CT |
12 F/6 M 18 to 69 yr Mean: 49.1 |
12-36 mo post loading Mean: 19 mo |
| Donos et al.9 (2019) |
Prospective, single blind RCT |
16 |
SLActive T: Immediately provisionalized with non-occluding temporary crown C: Left without a crown+GBR |
Esthetic area Ant/premolar (single tooth) |
Mean BL change from baseline:T: 12 mo: –0.62 mm (peak of bone loss) 36 mo: –0.42 mm 48 mo: –0.41 mm 60 mo: –0.42 mmC: 12 mo: –0.18 mm 36 mo: –0.10 mm 48 mo: –0.24 mm 60 mo: –0.37 mm 60 mo: similar bone loss. |
SVR: 100% SCR:C: 77.8% (36 mo), 88.9% (48 mo) & 66.7% (60 mo) Mean PD changes:T: Only limited changesC: more pronounced changes, more improvements in PES, soft tissue contour & mesial papilla Largest differences in PD change: 48 mo (T: 0.9 mm, C: 1.6 mm) & 60 mo (T: 1.43 mm, C: 2.2 mm). |
PA |
5 M/11 F Mean:48.9 (T) & 49.6 (C) |
36, 48 & 60 mo |
| Marković et al.10 (2015) | Prospective Clinical | 37 | SLActive |
Max Post |
Continuous & significant bone loss: 0.4 mm 0.5 mm or higher around 2 implants |
SCR: 100% Stability at baseline: 71.7 (increased to 1 yr [80.3], except at 2 wk with a nonsignificant decrease [71.9]) |
CBCT |
13 Mean age: 47.1 |
1 yr |
| Canullo et al.16 (2010) | RCT | 69 | Platform diameters:C: 3.8 mmT1: 4.3 mmT2: 4.8 mmT3: 5.5 mm +bone substitute |
Max Post |
Inverse correlation between the extent of mismatching & amount of bone loss. Inverse correlation between MBL & abutment-implant diameter. Mean bone loss: 21 mo: C: 1.49 mm T1: 0.99 mm T2: 0.82 mm T3: 0.56 mm33 mo: No difference with 21 mo data except for T2 (0.87 mm) & T3 (0.64 mm). |
No BOP PD <3 mm |
PA |
17 M/14 F Mean age: 52.1 |
9, 15, 21, 33 mo |
| Al-Nawas et al.1 (2012) | Double blind, prospective RCT | 178 |
A: Ti13Zr B: Ti Grade IV small-diameter, SLActive Overdenture(No graft) |
Man(interforaminal region) |
6 mo: most BL changes:A: –0.23 mmB: –0.23 mm 12 mo:A: –0.34 mmB: –0.31 mm |
No differences in PI & SBI. 12 mo:SVR: 98.9% (A) & 97.8% (B)SCR: 96.6% (A) & 94.4% (B) |
Panoramic |
91 Mean age: 65.8 |
6 & 12 mo |
| Puisys and Linkevicius6 (2015) | Prospective controlled clinical trial | 97 |
Vertical gingival thickness: T1: thin, 2 mm or less T2: thin thickened with allogenic membrane C: thick, >2 mm |
Man Post |
Bone loss:2 mo: T1: 0.75 mm mesially & 0.73 mm distally. T2: 0.16 mm mesially & 0.2 mm distally. C: 0.17 mm mesially & 0.18 mm distally.1 yr: T1: 1.22 mm mesially & 1.14 mm distally. T2: 0.24 mm mesially & 0.19 mm distally. C: 0.22 mm mesially & 0.20 mm distally. Significant differences between T1/T2 & T1/C mesially & distally. |
SVR: 100% | PA |
28 M/69 F Mean age: 47.3 |
2 mo Postloading& 1 yr |
| Nóvoa et al.22 (2017) | Clinical trial | 60 |
SLActive Abutment heights:C: 1 mm T: 2.5 mm |
NM |
Bone loss up to:C: 1.3 mm T: 0.33 mm Mean BL change:1 yr: C: 0.82 mm T: 0.2 mm2 yr: C: 1.27 mm T: 0.22 mm3 yr: C: 1.23 mm T: 0.35 mm |
- | PA | - | 1, 2 & 3 yr |
(BL: bone level, Max: maxilla, Man: mandible, Ant: anterior, Post: posterior, MBL: marginal bone loss, SVR: survival rate, PA: periapical, F: female, M: male, SCR: success rate, GBR: guided bone regeneration, CBCT: cone-beam computed tomography, RCT: randomized clinical trial, HA: hydroxyapatite, b-TCP: beta-tricalcium phosphate, T: test, C: control, PD: probing depth, BOP: bleeding on probing, BIC: bone-implant contact, PES: pink esthetic score, SBI: sulcus bleeding index, DIM: distance from the mucosal margin to the implant shoulder, ICAI: implant crown aesthetic index, NM: not mentioned, Ti13Zr: Titanium13 Zirconium, DIB: distance from implant shoulder to the first BIC, PI: plaque index)
Table 2
| Study |
Study type |
No. of implants |
Study design |
Implant placement area |
Bone results | Other results | Measurement device |
Sex & age (yr) |
Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Kang et al.25 (2018) | Retrospective radiographic observational | 1,692 |
GBR in 7.7% Sinus graft in 6.7% |
Max/Man Ant/Post |
Overall bone loss:3 yr: 0.07 mm5 yr: 0.09 mm7 yr: 0.14 mm9 yr: 0.17 mm 14 implants with pathologic MBL >2 mm. 2 implants were removed with progressive MBL (5.5 & 7.5 yr). 5 implants showed early bone loss >1 mm within 1st yr but then showed a stable MBL. In 7 implants, bone loss started after 1st yr & progressed continuously. Implant diameter affected MBL. >99% of implants showed <1 mm bone loss in 3 yr, 1.9% >1 mm bone loss. Implants with >3 mm bone loss: only after 5 yr. |
SVR: 98.2% |
Panoramic PA |
881 (496 M/385 F) Mean age: 52.2 |
10 yr Mean: 5.3 yr |
| Buser et al.35 (2012) | Retrospective | 511 | SLActive |
Max/Man Ant/Post Partially edentulous |
Sufficient BV in 70% of implant sites. 17.6% of implants had insufficient crest width. Mean DIB: 3.32 mm DIB: 49.5%: between 2.51 & 3.50 mm (no or minimal bone loss). 11.3% <2.5 mm (no bone loss or gain). 34.9%: between 3.51 & 4.5 mm (moderate bone loss). 4.4% >4.51 mm. Latter two subgroups: narrow radiolucent gap along implant surface in crest. |
SVR: 98.8% SCR: 97.0% Mean PI: 0.65 Mean PD: 3.27 mm Mean SBI: 1.32 Mean DIM:–0.42 mm. |
PA |
303 (160 F, 52.8%/143 M, 47.2%) Mean age: 48 |
10 yr |
| Friedmann et al.26 (2011) | Randomised controlled, single-blinded pilot clinical trial | 73 |
Lateral augmentation & GBR Biphasic CaP+ membranes: T: ribose cross linked coll membranes C: non-cross- linked mem branes |
Max/Man Ant/Post |
Gain in clinically hard MT at crestal level: T (lateral defects): 1.8 mm C (lateral defects): 0.7 mm T (vertical defects): 1.1 mm C (vertical defects): 0.2 mm Second measurement: Lateral defects:(median width gain): T: 3.0 mm C: 2.1 mm (median vertical gain): T: 2.5 mm C: 2.7 mm |
SVR: 100% Soft tissue dehiscences at 70.5% & 55% frequency for T & C |
Morphometric |
37 Mean: 53 |
6 mo |
| Ladwein et al.32 (2015) | Clinical cross-sectional analysis | 967 |
A: NKM B: KM |
Max/Man Ant/Post |
No difference in vertical BL. Of Post implants, 40.3% showed NKM. Of Ant implants, 30.4% showed NKM. |
Mean KM width: 1.87 mm. A: more PI & SBI No difference in PD: PD mesial A: 3.78 mm B: 3.61 mm |
Panoramic |
211 (97 M/114 F) Mean: 54.63 (maximum:78) |
Mean: 7.78 yr (4-15 yr) |
| Le and Borzabadi-Farahani44 (2014) | Clinical trial | 156 |
Transmucosal implant/Simultaneous GBR (allograft) Vertical defect: A: small (<3 mm) B: medium (3-5 mm) C: large (>5 mm) |
Max/Man Post Localized buccal wall of bone defects |
Significant differences in simultaneous grafting with different pre-treatment vertical defect sizes. Two graft failures (one needed regrafting) & 2 implant failures. Complete correction of 100% & 79.3% of A & B. C: only partial improvement in 90% of cases, without any complete correction. |
SVR: 98.1% | CBCT |
108 (38 M/70 F) Mean: 46.7 |
36 mo |
| Fretwurst et al.42 (2015) | Retrospective | 150 | Onlay graft (anterior superior iliac crest) |
Max/Man Partially edentulous/edentulous with severe alveolar ridge resorption & remaining BV of <5 mm in height |
Mean crestal bone loss: 10 yr: 1.8 mm (>5 mm increase). Significant difference between sex & crestal bone loss, but no influence of implant system, diameter, & patient age. 10 yr mean BL change: F: 2 mm (range, 0.5-4 mm) M: 1 mm (range, 0.5-2 mm) |
SVR: Max: 96% Man: 92% Total: 95% |
Panoramic |
32 (22 F/10 M) Mean age: 52 |
Mean: 69 mo (range, 12-165 mo) (Graphy at 1, 3, 5, 10 yr) |
| Agustín-Panadero et al.27 (2019) | Prospective observational | 42 |
A: convergent transmucosal collar B: divergent collar |
Max/Man Post (molar & premolar) |
Mean bone loss (total) (significant difference):A: 0.29 mmB: 0.6 mm Mesial areas (No significant difference):A: 33.3% (0.32 mm)B: 47.6% (0.42 mm) Distal areas (significant difference):A: 38.1% (0.26 mm)B: 66.7% (0.78 mm) Mean bone loss:Man (significant difference): A: 0.19 mm B: 0.72 mmMax (similar) A: 0.36 mm B: 0.51 mm A: Same bone loss in both jaws regardless of areas. |
- | PA | 21 |
2 yr Postloading |
| Buser et al.45 (2013) | Prospective, cross-sectional | 41 | GBR |
Max Ant(central to premolar)(single tooth) |
PA: Stable peri-implant BL & mean DIB: 2.18 mm. CBCT: mean thickness of facial bony wall: 2.2 mm & mean thickness from 1.58 to 2.33 mm. 85% of implants: bone loss or bone gain within –0.8 & 0.8 mm. 4.9% of implants had no facial bony wall. |
Mean PES: 7.49 Mean WES (more stable than PES): 6.88 Mean PD: 4.26 mm DIM: –3.42 mm (1st examination) & –2.21 mm (2nd examination) |
CBCT PA |
41 (25 M/16 F) Mean age: 38.8 |
5 to 9 yr (mean: 7 yr) |
| Canullo et al.34 (2020) | Prospective | 16 | Convergent collar |
Max Ant |
Mean BL change: 0.071 mm |
SVR: 100% PES (mean): Mesial papilla: 1.69 Distal papilla: 1.81 Total: 8.5 |
PA |
15 (11 M/4 F) Mean age: 54.6 |
3 yr |
| Makowiecki et al.28 (2017) | Comparative preliminary | 30 |
T: short with hydrophilic surfaces C: SLActive(early & delayed loading) |
Man Post |
3 mo:Significant difference in primary stability & MBL. C (higher MBL): 0.53 mm T: 0.37 mm 6 mo: No significant difference in secondary stability. C: 0.57 mm T: 0.51 mm C: No differences in MBL between 3 & 6 mo. |
- | CBCT |
Mean age: T: 36 C: 45.5 |
12 & 24 wk |
(TL: tissue-level, GBR: guided bone regeneration, Max: maxilla, Man: mandible, Ant: anterior, Post: posterior, MBL: marginal bone loss, SVR: survival rate, PA: periapical, M: male, F: female, BV: bone volume, DIB: distance from shoulder to the first bone-implant contact, SCR: success rate, PI: plaque index, PD: probing depth, SBI: sulcus bleeding index, DIM: distance from the mucosal margin to the implant shoulder, CaP: calcium-phosphate, T: test, C: control, MT: mineralized tissue, NKM: nonkeratinized mucosa, CBCT: cone beam computed tomography, BL: bone level, WES: white esthetic score, PES: pink esthetic score)
Table 3
| Study | Study type | No. of implants | Study design |
Implant placement area |
Bone results | Other results | Measurement device |
Sex & age (yr) |
Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Kumar et al.29 (2014) | Retrospective clinical | 337 |
BL: 179 TL: 158 |
Max/Man Ant/Post |
12, 24, 36 mo: Mean MBL: BL: 0.3, 0.38, 0.48 mm TL: 0.6, 0.54, 0.9 mm No significant difference at 6-12 mo & slightly greater in TL. Deeper implants showed more bone loss. IDIP: BL: implant shoulder very near crestal bone (range, −0.71 to +0.78 mm; mean, +0.007 mm) TL: shoulder 0.43 to 2.73 mm above crestal bone margin (mean, 1.65 mm) |
- | Panoramic | 129 | 12, 24, & 36 mo |
| Chiapasco et al.41 (2012) | Prospective | 51 |
TL: 13 BL: 38 Autogenous vertical onlay grafts from calvarium or ramus |
Max/Man Ant/Post(horizontally deficient edentulous ridge) |
Mean bone resorption: 0.52 mm (0-1 mm) in constructed areas0.41 mm in calvarial grafts Mean bone resorption prior to implant placement: 0.18 mm for calvarial & 0.42 mm for ramus grafts |
SCR:90.3% (calvarial grafts)93.1% (ramus grafts) SVR: 100% |
Panoramic PA |
18 (6 M/12 F) Mean: 49.1 |
12-36 mo(mean, 19 mo) |
| Chiapasco et al.40 (2014) | Retrospective | 192 |
TL: 97 BL: 95 Autogenous onlay grafts (ramus, iliac, calvaria) |
Max/Man Ant/Post Vertical or 3D defects of edentulous ridges |
Mean bone resorption: TL: 0.23 mm in ramus grafts, 0.36 mm in iliac grafts, 0.35 mm in calvarial grafts. BL: 0.48 mm in ramus grafts, 1.34 mm in iliac grafts, 0.35 mm in calvarial grafts |
SVR: 100% SCR: TL: 100% BL: 86.8%(93.5% in ramus grafts, 90.3% in calvarial grafts & 76.4% in iliac grafts) Overall complications: TL: 0% BL: 5.4% |
Panoramic PA |
50 (16 M/34 F) Mean age: 49.5 |
12-68 mo postloading(mean, 33 mo) |
| Lopez et al.30 (2016) | Retrospective cohort | 150 |
Cylindrical 76 in F 74 in M GBR |
Max/Man Ant/Post |
Mean MBL: 92%. Mean bone loss: 0 mm Mean bone loss:BL: 0.12 mm TL: 0.04 mm |
SVR: 98.7% SCR: 92% |
Panoramic PA |
Mean age: 60 | Mean: 84 mo |
| Vianna et al.47 (2018) | Prospective, split-mouth RCT | 40 |
TL: 20 BL: 20 |
Max/Man Ant/Post |
Mean MBL up to 24 mo: TL: 0.75 mmBL: 0.70 mm Implant insertion:TL: 1.48 mmBL: 0.08 mm Prosthesis Installation:TL: 2.22 mmBL: 0.67 mm 6 mo:TL: 2.32 mmBL: 0.62 mm 24 mo:TL: 2.14 mmBL: 0.77 mm |
No significant difference for PI & BOP. 80% of sites in both with at least one bleeding site at 12 mo & 90% at 24 mo. Similar PD |
CBCT PA |
20 (with history of chronic periodontitis)(6 M/14 F)Mean age: 49.13 |
Implant insertion, Prosthesis installation, 6 & 24 mm postloading |
| Fernández-Formoso et al.39 (2012) | RCT | 114 |
TL: Standard matched BL: Platform switched |
Max/Man Post |
Mean bone loss (significant difference): BL: 0.01 mm TL: 0.42 mm Mean of DIB (significant difference between groups): TL: 0.42 mm (significant difference) BL: –0.01 mm (no significant difference) |
- | PA |
54 TL: 25 (16 F/9 M) Mean age: 43.7 BL: 26 (17 F/9 M) Mean age: 42.9 |
1 yr |
| Lago et al.38 (2018) | RCT | 197 |
TL: Platform matched BL: Platform switched |
Max/Man Post |
Mean MBL:TL: Baseline to 1 yr: 0.26 mm 1 to 5 yr: 0.34 mm Baseline to 5 yr: 0.61 mm BL: Baseline to 1 yr: −0.03 mm 1 to 5 yr: −0.17 mm Baseline to 5 yr: −0.20 mm Significant difference between groups: Baseline to 1 yr: 0.31 mm 1 to 5 yr: 0.53 mm Baseline to 5 yr: 0.85 mm |
SVR: TL:1 yr: 100%5 yr: 98% BL:1 yr: 99%5 yr: 96.1% |
CBCT PA |
54 M/46 F Mean age: 50.5TL: 50 (31 M/19 F) Mean age: 47.9BL: 50 (23 M/27 F) Mean age: 53.1 |
1 & 5 yr after definitive restoration |
| Lago et al.37 (2019) | Split-mouth RCT | 100 |
TL: Platform matched BL: Platform switched |
Max/Man Post |
Crestal bone changes: Baseline to 3 yr:BL: 0.18 mmTL: 0.15 mm Mean:Baseline to 1 yr: 0.07 mm1 to 3 yr: 0.01 mmBaseline to 3 yr: 0.04 mmOnly significant difference in TL from baseline to 3 yr. |
- | PA |
35 (15 M/20 F) Mean: 49.5 |
1 & 3 yr after definitive restoration |
| Wallner et al.33 (2018) | Clinical trial | 42 |
TL: 20 BL: 22 |
Max Ant |
1.9 yr: BL: 14 implants with thick biotype & mean bone change of –0.03 mm & 8 with thin biotype & change of 0.09 mm. Total mean bone change: +0.02 mm 4.9 yr: TL: 12 implants with thick biotype & mean bone loss of 0.21 mm & 8 with thin biotype & mean bone loss of 0.05 mm. Total mean bone loss: 0.015 mm |
- | PA |
Human 41 (28 F/13 M) TL: mean age, 39 BL: mean age, 45 |
Mean:4.9 yr (11 mo to 7.8 yr) |
| Hadzik et al.31 (2017) | Comparative | 32 |
Short implants BL: 16 TL: 16 |
Man (lateral aspect) |
MBL:BL<TL12 & 36 wk (significant difference in MBL): BL: Significant increase (0.19 & 0.29 mm or about 50%). TL: No significant changes (0.53 & 0.57 mm). |
Primary stability:BL: 77.8TL: 66.5 Secondary stability: BL: 78.9TL: 73.9 |
PA CBCT |
13 A: 7 (mean age, 45.9) B: 6 (mean age, 46.3) |
12 & 36 wk |
(BL: bone level, TL: tissue level, Max: maxilla, Man: mandible, Ant: anterior, Post: posterior, MBL: marginal bone loss, IDIP: initial depth of implant placement, SCR: success rate, SVR: survival rate, PA: periapical, M: male, F: female, GBR: guided bone regeneration, RCT: randomized clinical trial, PI: plaque index, BOP: bleeding on probing, PD: probing depth, CBCT: cone-beam computed tomography, DIB: distance from shoulder to the first bone-implant contact)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download